Introduction
Window flies (Diptera: Scenopinidae) are a cosmopolitan family of asiloid flies comprising over 420 known species distributed in 25 genera worldwide, several of which have significant continental endemism (Winterton & Gaimari 2011, Winterton & Ware 2015). Three subfamilies are recognized, Caenotinae (one genus), Proratinae (six genera), and Scenopininae (18 genera), the latter with five endemic genera belonging to the New World, Belosta Hardy, Brevitrichia Hardy, 1944, Heteromphrale Kröber, 1937, Irwiniana Kelsey, 1971 and Pseudatrichia Osten Sacken, 1877 (Kelsey 1973, Winterton & Gaimari 2017).
In the Neotropical region, seven genera are found containing only 24 species: Brevitrichia, Irwiniana, Jackhallia Nagatomi & Liu, 1994, Pseudatrichia, Heteromphrale, Metatrichia Coquillett, 1900 and Scenopinus Latreille, 1802 (Winterton & Gaimari 2017, Rafael et al. 2022). Many more species are expected to be discovered in this region as many countries remain poorly sampled for most dipteran groups, like Peru where only one species is known to occur, Scenopinus magnicornis (Kröber, 1913) (Kelsey 1969, 1973, Amorim 2009).
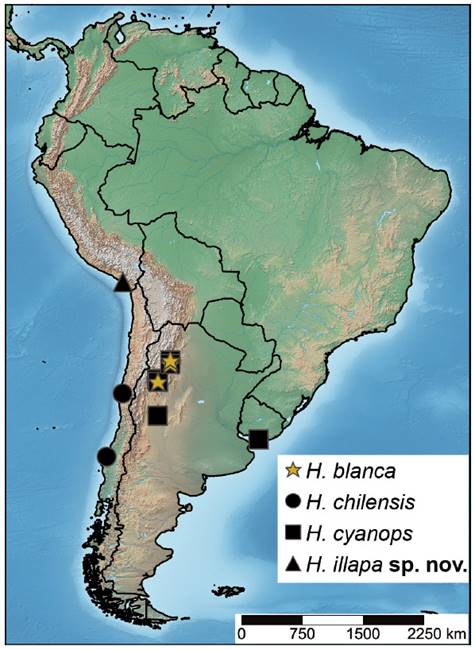
Heteromphrale is a Neotropical genus known from Argentina, Chile, and Uruguay, comprising three extant species: Heteromphrale blanca (Edwards, 1932), Heteromphrale chilensis (Kröber, 1937), and Heteromphrale cyanopsWinterton & Gaimari, 2011 (Winterton & Gaimari 2011). This genus can be differentiated from Brevitrichia (the closest related genus) by the relatively short and straight distiphallus and subepandrial sclerite not modified, and the straight or slightly emarginate apically sternite 8 in females (Winterton & Gaimari 2011, Winterton & Gharali 2011). In this work, we report Heteromphrale for the first time from Peru and describe a new species, increasing the number of species in this genus to four. A distribution map and an updated key to species of Heteromphrale are provided.
Material and methods
Specimens included in this work are deposited in the entomological collection of the Natural History Museum, Universidad Nacional Mayor de San Marcos, Lima, Peru (MUSM). Digital images of specimens were taken with a Leica MC 190 HD digital camera mounted on a Leica SAPO stereoscopic microscope, and then integrated as single images with Zerene stacker software (Zerene Systems LLC).
The genitalia were briefly heated in 10 % KOH to remove soft tissue, then rinsed in distilled water, treated with 10 % acetic acid, and examined in concavity slides with glycerine. Lastly, the detached parts were stored in polyethylene microvials with glycerine for preservation and pinned with their respective specimens. Morphological terminology follows Winterton and Gaimari (2011), and like these authors structures of the male terminalia (rotated 180° in Scenopininae) are described and labelled as they are in related flies with terminalia not rotated (i.e., the ventral apodeme of the aedeagus is physically located dorsally).
The type data are transcribed in full, forward slashes separate different labels from the same specimen and square brackets enclose complementary or explanatory information not included in labels. Distribution maps were elaborated with SimpleMappr (Shorthouse 2010).
Taxonomy and descriptions
Heteromphrale Kröber, 1937
Heteromphrale illapa sp. nov.
(Figs. 1 - 2)
Material examined. Holotype: ♂, PERU: MO ENT#091;MoqueguaENT#093;, Ilo, El Algarrobal, 17°34’45.85”S, 71°9’42.41”W, 768 m, 25.ii.2023, G, Saravia ENT#091;Moericke trapENT#093;/ HOLOTYPE ♂ Heteromphrale illapa Sánchez & Ramírez-Montano (MUSM). Paratypes. 2♀, same data as holotype / PARATYPE ♀ Heteromphrale illapa Sánchez & Ramírez-Montano (MUSM).
Distribution. Moquegua, Peru. The type locality is located in the coastal desert of southwestern Peru.
Holotype condition. Good, detached terminalia stored in glycerine and pinned along with specimen.
Diagnosis. Antenna dark brown, flagellum abruptly pear-shaped; mouthparts smaller than oral cavity; scutum without glabrous dorsocentral patches, but female presents a glabrous and glossy central stripe on anterior half; wing with vein R4 diverging from R5 at point in basal quarter of cell r5 (anterior to apex of discal medial cell); abdomen dark brown with light brown (male) to yellow (female) posterior bands on tergites 2-5, more extensive and conspicuous in female; tergite 2 sensory patch distinct as two small patches; male epandrium with posterior margin rounded; hypandrium small with a single lobe; distiphallus arms divergent; lateral aedeagal bulbs relatively small; female sternite 8 posteriorly attenuate, more or less pointed; acanthophorite spines long and stout.
Description of male holotype. (Fig. 1 a-b) Body length: 2.5 mm, wing length: 1.6 mm
Head. (Fig. 1c) Brownish yellow, except narrow line along eye margin on upper frons and ocellar triangle, dark brown; eyes contiguous, separated at vertex by a little more than width of ocellar triangle; ocellar triangle gray pubescent, raised, with anterior ocellus slightly larger than posterior ones; scape and pedicel dark brown, the latter with minute pale setae apically; flagellum dark brown to black, abruptly pyriform, tapered on about distal half; mouthparts relatively small in size, smaller than oral cavity, brownish yellow except basally, darkened. Thorax. Scutum black, brownish yellow on apical margin of postpronotal lobe, anterior part of scutum adjacent to postpronotal lobe, lateral margin of scutum and postalar callus; predominantly overlain by dense glaucous pubescence and sparse dorsocentral and acrostichal pale setae; scutellum black medially, yellowish on lateral margins and brownish on posterior margin; pleura mainly brown to black, except on upper margin, yellowish; coxae and legs brown, white setose. Wing. Venation pale brown; vein R4 diverging from R5 at point in basal quarter of cell r5 (anterior to apex of discal medial cell); haltere stem yellow, knob white. Abdomen. Segments dark brown with posterior light brown bands that stand out little from the background; pale setae laterally, very few on posterior margins of tergites; tergite 2 sensory patch large and distinct, dark brown concolorous with anterior band. Terminalia. (Fig. 1 d-g). Epandrium yellowish brown, subrectangular with posterior margin rounded, white setae marginally; hypandrium halves small, subconical and encircling a single rounded lobe; dorsal process of gonocoxite acuminate; gonostylus large, apparently fused to gonocoxite, extending little beyond to the apex of distiphallus and medially united; gonocoxal apodeme more or less pointed, flattened dorsoventrally, gently directed inwards (outer margin curved, inner margin nearly straight); ejaculatory apodeme small, narrow in lateral view and directed downwards (physically upwards); lateral aedeagal bulbs small; ventral apodeme dark sclerotized; distiphallus divergent laterally and gently curved downwards around gonostylus; subepandrial sclerite, curved on second third, apex spatulate and rounded.
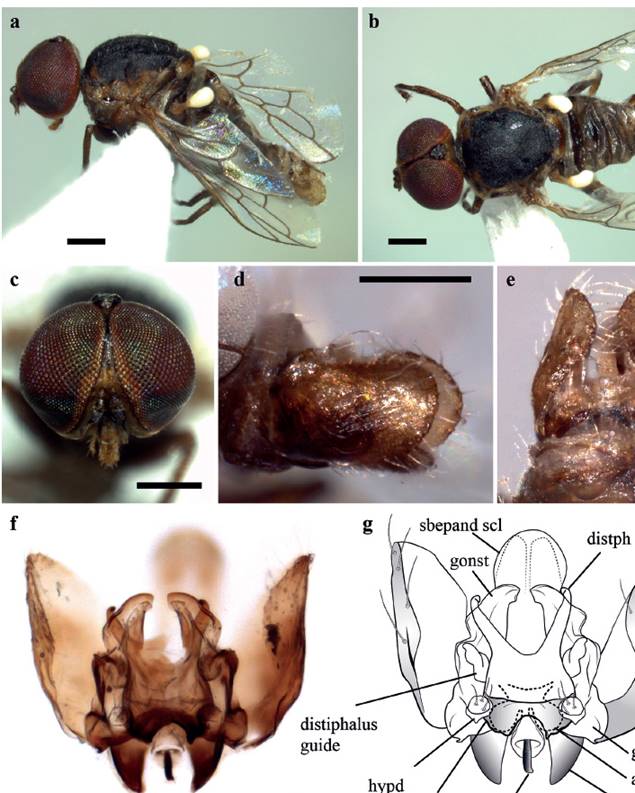
Figure 1 Heteromphrale illapa sp. nov. Male. a - b. Habitus, lateral and dorsal views, c. head, frontal view, d - e. Terminalia, lateral and ventral (physically dorsal) views, f - g. Terminalia after treatment with KOH, ventral view. Abbreviations. aed lb - aedeagal lobe; distph - distiphallus; ej apod - ejaculatory apodeme; epand - epandrium; goncx - gonocoxite; goncx apod - gonocoxal apodeme; gonst - gonostylus; hypd - hypandrium; sbepand scl - subepandrial sclerite; v apod - ventral apodeme of parameral sheath. Scale bar = 0.25 mm.
Female. (Fig. 2 a-b) Body length: 4.5 mm, wing length: 2.8 mm.
Larger than male, differing from this by the following: Head. (Fig. 2c). Almost entirely cream yellow, except ocellar triangle, dark brown and upper frons and upper occiput with tan suffusion on sides; eyes dichoptic, separated at vertex by about twice the width of ocellar tubercle; frons furrowed medially, dividing it in two protruding glabrous areas; mouthparts yellow. Thorax. Scutum black to gray and cream yellow, yellow color more extensive than in male, especially on postpronotal lobe (with only a black spot basally) and posterior third of scutum, as two narrow dorsocentral stripes; thorax overlain with dense glaucous pubescence and pale setae except by a glabrous and glossy central stripe on anterior half of scutum; scutellum black medially, cream yellow on lateral margins and brownish on posterior margin; pleura cream yellow with black spots on the lower part of anepimeron and upper part of katepisternum and meron; prosternum yellow; coxae yellow, with brown suffusion; legs yellow with mottled brown suffusion on femora and tibiae, tarsi darkened apically. Wing. Haltere pale yellow. Abdomen. Posterior bands of abdominal segments cream yellow, more extensive than in male and clearly standing out from the background. Terminalia. (Fig. 2 d-i). Sternite 8 posteriorly attenuate, more or less pointed; acanthophorite spines long and stout; two sclerotized spermathecae, bean-shaped, located basally in abdominal segment 6; genital fork sclerotized, connected posteromedially with “Y” shaped sclerotized bridge between genital fork and anterior margins of tergites 9 and 10.
Etymology. The specific epithet is a masculine noun in apposition. Illapa was the Inca god of thunder, lightning and rain.
Taxonomic discussion. The most similar species to H. illapa sp. nov. is H. chilensis. Externally, both species share the mouthparts smaller than oral cavity and the basal antennal flagellomere abruptly pear-shaped. In addition, a more evident relation can be observed in the male genitalia, very similar, especially in the distiphallus, which presents arms divergent laterally, while in the other two species of Heteromphrale it presents parallel arms. However, H. illapa sp. nov. can be easily differentiated from H. chilensis because this latter species presents the abdomen distinctly matte white and its female has the frons extensively pilose, while in H. illapa sp. nov. the abdomen is more extensively brown and the female frons lacks pile. Additionally, in H. chilensis the scutum of both sexes presents glabrous and glossy dorsocentral areas posteriorly (circular in male, linear in female), something that can only be observed in the female of H. illapa sp. nov., although it presents a single linear glossy area of central position on the anterior half of the scutum. On the other hand, regarding the male genitalia, the most significant difference can be observed in the hypandrium halves, with a single lobe in H. illapa sp. nov. (Fig. 1f-g) and multiple lobes in H. chilensis.
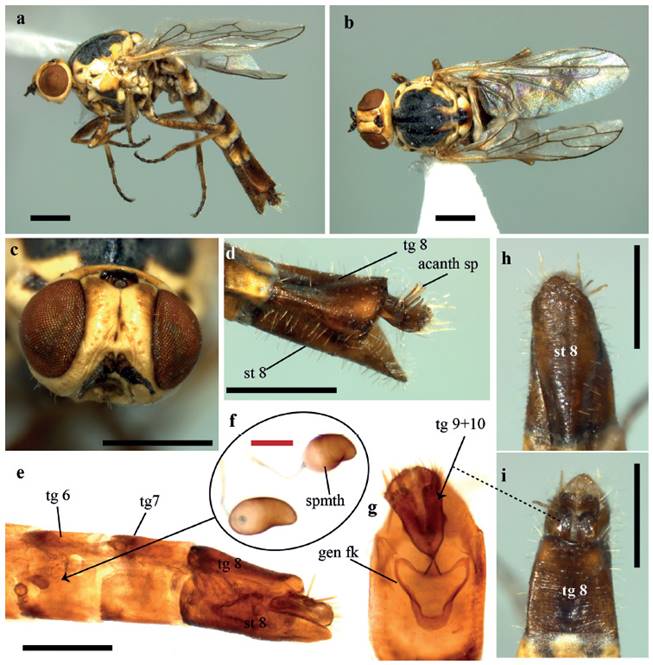
Figure 2 Heteromphrale illapa sp. nov. Female. a - b. Habitus, lateral and dorsal views, c. head, frontal view, d. Terminalia, lateral view, e - f. Terminalia after treatment with KOH, lateral and ventral views (Showing positions of spermathecae and genital fork). h - i. Terminalia, ventral, and dorsal views. Abbreviations. acanth sp - acanthophorite spines; gen fk - genital fork; spmth - spermatheca; st - sternite; tg - tergite. Scale bars; black = 0.5 mm, red = 0.1 mm.
Key to Heteromphrale species (Modified fromWinterton and Gaimari, 2011)
1 Scutum with glabrous, glossy area (at least in female) (Fig. 2b and Fig. 5C, D in Winterton & Gaimari 2011); basal antennal flagellomere abruptly pear-shaped; mouthparts smaller than oral cavity; legs, at least in female, with mottled brown suffusion; distiphallus with arms divergent laterally (Fig. 1g); lateral aedeagal bulbs relatively small 2
- Scutum with uniform covering of pubescence, lacking a glabrous or glossy mark; basal antennal flagellomere more conical-shaped, tapering evenly; mouthparts usually normal-sized, nearly filling oral cavity; legs, if brown suffusion present, not mottled; distiphallus with arms parallel; lateral aedeagal bulbs large 3
2 Female frons with extensive pile, abdomen distinctly matte white with brown suffusion laterally and ventrally; hypandrium with multiple lobes directed posteromedially; female sternite 8 rounded posteriorlyH. chilensis (Kröber) (Chile)
- Female frons without pile; abdomen in both sexes more extensively brown; hypandrium with a single lobe directed posteromedially; female sternite 8 pointed posteriorlyH. illapa sp. nov. (Peru)
3 Wing with vein R4 diverging from vein R5 at point in basal quarter of cell r5 (anterior to apex of discal medial cell) (See Fig. 8B in Winterton & Gaimari 2011); tergite 2 sensory patch as relatively small single patch, slightly narrowed; male epandrium enlarged, bulbous, without distal fringe of long, white setae on posterior edge (See Figs. 3A-B, 8A, 10C in Winterton & Gaimari 2011); female sternite 8 shallowly emarginate posteriorly and without a fringe of long setae; acanthophorite spines stout (See Fig. 9G-H in Winterton & Gaimari 2011)H. cyanops (Edwards) (Argentina and Uruguay)
- Wing with vein R4 diverging from vein R5 at point between one-quarter and one-half of cell r5 (posterior to apex of discal medial cell) (See Fig. 6 A, B in Winterton & Gaimari 2011); tergite 2 sensory patch large and distinct, divided into two small patches with setae directed medially; male epandrium not bulbous, size subequal to preceding abdominal segment, with distal fringe of long white setae on posterior edge (See Figs. 2A-B, 10A in Winterton & Gaimari 2011); female sternite 8 distally rounded with dense long thin setae apicolaterally and distally; acanthophorite spines thin and wispy (See Fig. 9A-C in Winterton & Gaimari 2011) H. blanca Winterton & Gaimari (Argentina)
Discussion
The 25 species of known scenopinids in the Neotropical region represent only 6% of the world extant fauna, something that reflects the poor knowledge of dipterans inhabiting this region that includes some countries where very little is known for most part of their territories, like Peru (Amorim 2009). Hence, although the Peruvian fauna of window flies cannot be expected to be large, the small number of species known to occur in this country certainly underestimate its actual diversity, which represents a challenging issue due to the small size of the members of this group that are usually overlooked in faunistic studies and whose material is relatively rare in collections.












 uBio
uBio