Introduction
In South American fishes, fourteen species of Monogenoidea have been documented parasitizing the Auchenipteridae (Siluriformes) family. These parasites are attributed to the genera Demidospermus Suriano, 1983, and Cosmetocleithrum Kritsky, Thatcher, and Boeger, 1986. The reported occurrences are primarily concentrated in Brazil, they are: Demidospermus bidiverticulatum Suriano & Incorvaia, 1995, and D. osteomystax Tavernari, Takemoto, Lacerda & Pavanelli, 2011 were found parasitizing Auchenipterus osteomystax (Miranda-Ribeiro, 1918) (Cohen et al., 2013); D. tocantinensis Cohen, Justo, Gen & Boeger, 2000, and D. osteomystax Cohen, Justo, Gen & Boeger, 2000 parasitized A. nuchalis (Spix & Agassiz, 1829) (Cohen et al. 2020). Additionally, Cosmetocleithrum striatuli Abdallah, Azevedo & Luque, 2012 in Trachelyopterus striatulus Steindachner, 1877 (Cohen et al. 2013); C. bulbocirrus Kritsky, Thatcher & Boeger, 1986 in Ageneiosus ucayalensis Castelnau, 1855 (Soares et al., 2018); C. laciniatum Yamada, Yamada, Da Silva & Dos Anjos, 2017 in T. galeatus (Yamada et al., 2017); C. berecae Cohen, Justo, Gen & Boeger, 2020, and C. nunani Cohen, Justo, Gen & Boeger, 2020 in A. nuchalis; C. spathulatum Yamada, Yamada & da Silva, 2020; C. baculum Yamada, Yamada & da Silva, 2020, and C. galeatum Yamada, Yamada & da Silva, 2020 in T. galeatus (Yamada et al., 2020). In Argentina, D. uncusvalidus Gutiérrez & Suriano, 1992 in T. galeatus (Linnaeus, 1766) has been reported, and in Peru, D. centromochli Mendoza-Franco & Scholz, 2009 in Centromochlus heckeliim (DeFilippi, 1853) has been reported. Until now, in Peru, only one species of Auchenipteridae (C. heckeliim) has been recognized as a host for monogenoideans. During a study on monogenoideans infecting fishes from the Pintuyacu stream, Loreto, a novel species belonging to Cosmetocleithrum was discovered parasitizing the gills of Auchenipterichthys coracoideus Eigenmann and Allen, 1942. The new species is described herein based on morphological characteristics (Mendoza-Palmero et al. 2019).
Material and methods
Thirteen specimens of A. coracoideus, with 7.1 ± 0.5 cm of standard length and 3.2 ± 0.9 g of weight, were obtained in July 2019, from the Pintuyacu stream, which is a tributary of the Itaya River, in Iquitos, Loreto, Peru (04°05´59.4”S, 073°27´13.8”W).
The identification of fish hosts was conducted based on morphological characteristics following the methodology outlined by Carl et al. (2005). Live fish specimens were temporarily stored in plastic bags containing water and oxygen and transported to the "Laboratorio de Parasitología y Sanidad Acuícola" at the "Instituto de Investigaciones de la Amazonía Peruana" for parasitological examination. Gill arches were carefully removed and placed in vials filled with hot water (65 °C).
Subsequently, each vial was vigorously shaken, and 96% ethanol was added to preserve the samples. In the laboratory, the contents of each vial were examined under a stereomicroscope, and helminths were extracted from the gills or sediment using dissection needles. Some specimens were mounted on slides using Hoyer's medium to enhance visualization of soft tissues and sclerotized pieces. Additionally, other specimens were stained with Gomori's trichrome (Humason 1979, Boeger & Vianna 2006) and mounted in Canada balsam to facilitate observation of internal structures. Selected specimens were preserved in 96% ethanol for future DNA analyses. All procedures were conducted at the "Laboratorio de Parasitología y Sanidad Acuícola" of the "Instituto de Investigaciones de la Amazonía Peruana" (IIAP) in Iquitos, Peru.
Measurements of the monogenoideans were recorded in micrometers following the methodologies proposed by Mizelle and Klucka (1953). Dimensions of organs and other structures represent the maximum measurement in dorso-ventral view; lengths of curved or bent structures (anchors, bars, male copulatory organ [MCO], and accessory piece) denote straight-line distances between extreme ends. Mean values are presented alongside the range and the number (n) of specimens measured, in parentheses. Illustrations were prepared with the assistance of a microprojector.
Type specimens and vouchers were deposited in the Helminthological Collection of the Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos (MUSM), Lima, Peru. Ethical approval from an ethics committee and relevant licenses for working with fish species were obtained in accordance with the following resolutions: Resolution No132-2014-GRL-DIREPRO; Resolution No21-2016 GRL-DIREPRO; and PTH-068-16-PEC-SANIPES.
Taxonomy
Class Monogenea Van Beneden, 1858
Subclass Monopisthocotylea, Odhner, 1912
Order Dactylogyridea Bychowsky, 1937
Dactylogyridae Bychowsky, 1933
Cosmetocleithrum Kritsky, Thatcher & Boeger, 1986
Cosmetocleithrum amazonensis n. sp.
(Figures 1-10)
Type-host. Auchenipterichthys coracoideus (Eigenmann & Allen, 1942), (Siluriformes: Auchenipteridae).
Type-locality. Pintuyacu stream, Iquitos, Peru, July 2019 (04°05´59.4” S, 073°27´13.8” W).
Type-material. The holotype (MUSM-HEL 4423), 3 paratypes (MUSM-HEL 4422a-c) and 2 vouchers (MUSM-HEL 4422 d-e). The remaining 6 specimens were deposited in the collection of the “Laboratorio de Parasitología y Sanidad Acuícola” of the “Instituto de Investigaciones de la Amazonía Peruana” 6 voucher specimens (LPYSA M009a-f).
Site of infestation: Gill filaments.
Etymology. The specific name refers to the locality where the host and parasite were collected (Peruvian Amazon).
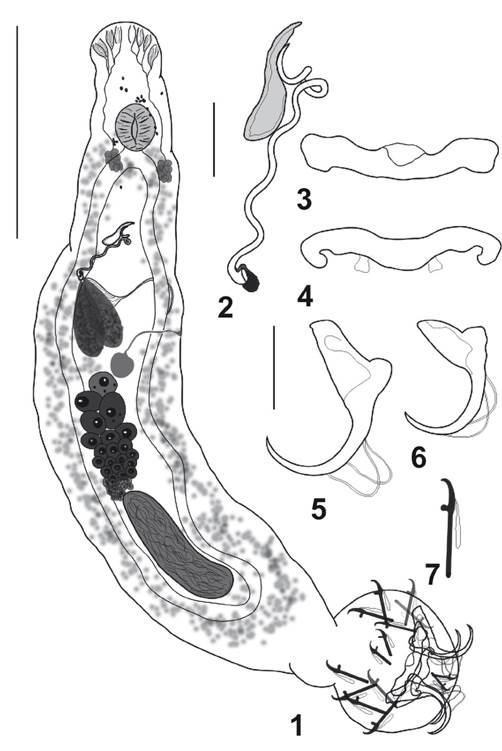
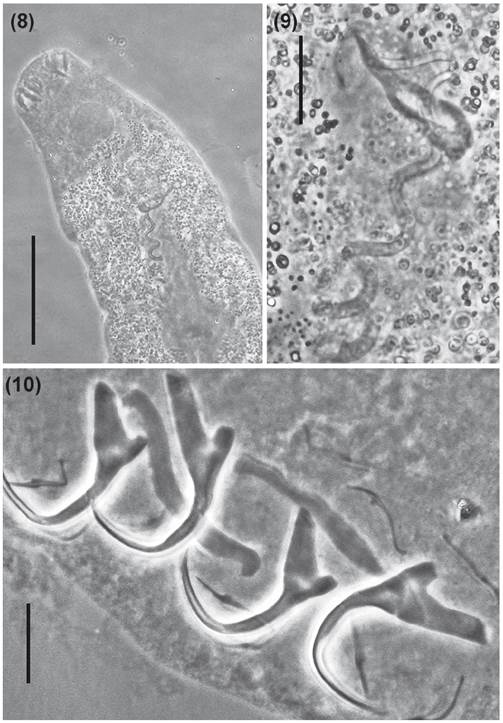
Figure 1 - 7 Cosmetocleithrum amazonensis n. sp. 1. Holotype, whole mount, ventral view; 2. Copulatory complex; 3. Ventral bar; 4. Dorsal bar; 5. Ventral anchor; 6. Dorsal anchor; 7. Hook pair 4. Scale bars: 1 = 150 µm. 2 - 4 = 10 µm; 5 - 7 = 15 µm.
Description
[Based on 12 specimens: 4 stained with Gomori’s trichrome and 8 mounted in Hoyer’s medium.] Body large and elongated, 543 (488-706; n = 12) long, greatest width 213 (119-301; n = 12), cephalic lobes poorly developed. Four pairs of head organs; cephalic glands unicellular, distributed lateral and posterolateral to the pharynx. Eyes inconspicuous; scarce accessory granules near the pharynx. Pharynx muscular, subspherical, 39 (38-54; n = 12) wide; esophagus short, two intestinal caeca, confluent posteriorly, lacking diverticula. Peduncle long, narrow; haptor, globose, 79 (53-115; n = 10) long, 115 (66-170; n = 12) wide. Ventral anchor 35 (23-38; n = 10) long, 36 (24-38; n = 10) wide, with well differentiated roots, superficial root much longer than deep root; curved shaft and point. Dorsal anchor 25 (20-27; n = 10) long, 27 (21-29; n = 10) wide, with robust roots. Superficial root longer than deep root; curved shaft and point. Ventral bar, 53 (43-55; n = 10) long, 12 (8-13; n = 10) wide, expanded laterally, with medial widening covered by a fold. Dorsal bar 44 (41-46; n = 10) long, 6 (4-8; n = 10) wide, with tapering ends and conspicuous widening along the shaft with two submedial short projections directed posteriorly, 4 (3-6; n = 10) long. Hooks 18 (15-19; n = 20) long, with Ancyrochephalinae distribution, similar in shape, erect thumb, straight shaft, short point; 5 ventral pairs, two dorsal pairs (hook pair 6 and 7). Testis dorsal, postgermarial, ovate; germarium ovate; vas deferens looping left of intestinal caecum; seminal vesicle elongate; two elongated prostatic reservoirs. Male copulatory organ 126 (82-132; n = 10) long, elongate sclerotized sinuous tube, with poorly defined coil, base with sclerotized fringe. Accessory piece sclerotized, 35 (25-54; n = 10) long, non-articulated to MCO, comprising a single plate. Seminal receptacle sac-like. Vagina sinistral, a thin and delicate elongated tube that joins the seminal receptacle. Eggs, ootype, uterus not observed. Vitellaria coextensive with caeca.
Remarks. Cosmetocleithrum amazonensis n. sp. share some characters common to all previously described species of Cosmetocleithrum: eyes absent, elaborate accessory piece not articulated to cirrus base and dorsal bar with two submedial projections directed posteriorly arising from anterodorsal surface of bar. Although Paracosmetocleithurm Acosta, Scholz, Blasco-Costa, Alves & Silva, 2018 also has these characteristics, species belonging to Cosmetocleithrum lack of a well-developed ornamentation in the middle portion of the ventral bar, and a sclerotized patch on the surface of the dorsal bar with an inconspicuous medial process. The new species resembles C. rarum Kritsky, Thatcher & Boeger, 1986 found in Trachelyopterus galeatus by presenting the MCO as an elongate sinuous tube and by the shape of the accessory piece that is a single plate. However, it can be distinguished from C. rarum and all other congeneric species by possessing a MCO quite long and sinuous, with the proximal region lying on the accessory piece and by presenting a ventral anchor with elongated superficial root.
Discussion
Twenty-two species of monogenoideans affiliated with Cosmetocleithrum have been documented in siluriform fishes, with 11 found in Doradidae species, one in Erythrinidae, one in Pimelodidae, and nine exclusively described in Auchenipteridae (Soares et al., 2018; Morey et al., 2019; Yamada et al., 2020). With the findings of our study, the tally of Cosmetocleithrum species increases to 23, and the count of monogenoidean species identified in auchenipterids rises from nine to ten.
In Peru, 14 genera and 28 species of Auchenipteridae have been documented (Ortega et al., 2012). To date, only one monogenoidean species has been recorded from an Auchenipteridae host collected in Peru. Our study presents the second documented instance of a monogenoidean species in an auchenipterid host from Peru.
The Peruvian Amazon remains relatively underexplored compared to countries like Brazil, but recent years have revealed it as a habitat for new monogenoidean species. Recent research, such as the description of five new species (Morey et al., 2019a; Morey et al., 2019b), including the most recent additions to the Cosmetocleithrum genus (Morey, Cachique & Babilonia, 2019), underscores this assertion. Given the substantial number of species yet to be investigated, the potential for discovering new monogenoideans is considerable.












 uBio
uBio