INTRODUCTION
C. parapsilosis has been considered a nosocomial pathogen for years, it frequently colonizes skin (it has been isolated in the hands of healthcare professionals) and adheres to synthetic material such as parenteral nutrition lines, intravascular catheters and other prosthetic devices by using biofilms1,2.
Among fungal endocarditis, C. parapsilosis represents the most common non-albican species isolated, and it is known to be an important cause of invasive candidiasis2,3. Heart valve surgery, intravenous drug consumption, prolonged antibiotic therapy, immunosuppression, previous episodes of endocarditis, and preexisting valve disease, are some of the main predisposing factors for C. parapsilosis infective endocarditis(CPIE)3,4. Of note, the most commonly affected heart tissue is the aortic valve, followed by the mitral and the tricuspid valve; also, in the outbreaks of CPIE, it has been found that intraoperative contamination is the most possible source of infection4.
In the literature there are some case reports of CPIE but most of these have imaging evidence of vegetations on the echocardiography. We describe the case of a middle-aged peruvian woman with history of heart valve surgery who presented to the emergency with a cardioembolic event due to a fungal endocarditis despite four echocardiographic procedures without evidence of vegetations or valve lesions. Treatment with high dose echinocandin was successful without the need of surgical intervention.
CASE REPORT
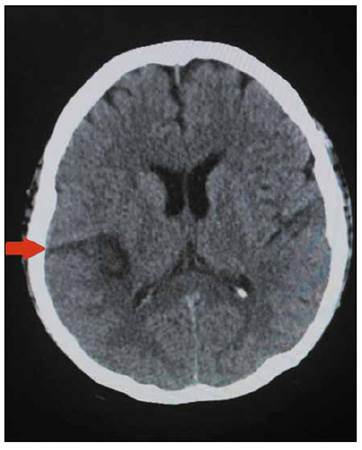
A 49-year-old female with past medical history of hypertension and severe aortic stenosis with subsequent aortic valve placement by modified Bentall procedure 8 months before, presented to the hospital with a seven-hour history of left arm and left leg numbness plus weakness and dysarthria. She was using warfarin since her prosthetic valve surgery. On admission, she complained of occipital headache and showed left hemiplegia on physical examination; no mental status changes or heart murmurs were auscultated. She was afebrile with a temperature of 36.5°C, a heart rate of 98 beats per minute, blood pressure of 140/90mmHg and respiratory rate of 18 breaths per minute. The brain computed tomography (CT) showed an ischemic stroke in the territory of the right middle cerebral artery (Figure 1), however no atherosclerosis was found on the carotid doppler ultrasound, and the trans-thoracic echocardiography (TTE) did not show vegetations. Additionally, the electrocardiogram was unremarkable.
Her white blood cell count was 5.48 cells/mm3, hemoglobin of 11.9 g/dL and she had a normal platelet count of 201 x 109/L. She was subsequently admitted to the neurology ward.
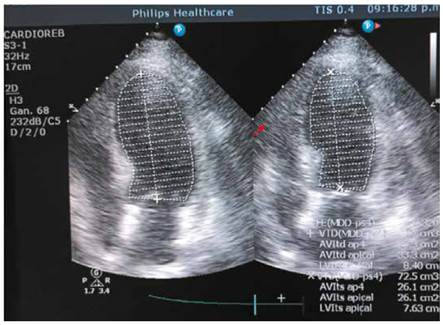
On the third day of hospitalization, she began with daily temperatures of 37.6°C that reached 38.9°C plus episodes of hypotension, she was started on empiric intravenous (IV) Vancomycin 1g/12h. plus Ceftriaxone 2g/day. Her urine analysis and chest radiograph where unremarkable, however she was still febrile, and her third set of blood cultures started growing yeast; the patient was started on IV Fluconazole 200mg/12h. Since a second TTE was normal, a transesophageal echocardiography (TEE) (Figure 2) was performed on the second week of hospitalization given the high suspicion of endocarditis but did not report any cardiac lesions including fistula or dehiscence. Blood cultures pending results confirmed the presence of C. parapsilosis in addition, a CT abdomen and eye examination were unremarkable.
During a 2-week period many infectious agents were tested including HBV, HCV, HIV, HTLV, tuberculosis and there were no markers of autoimmune disease. Despite antifungal therapy with IV fluconazole for almost 2 weeks, in which isolated C. parapsilosis was susceptible to all antifungals tested, except to ketoconazole according to the antifungal sensitivity test, MicroScan® (according to the FDA, is an automated system that provides results that are substantially equivalent to the generated using suitable reference methods)5 , the patient remained febrile, presented again episodes of hypotension and her INR reached 5.7. On the third week, in view of a possible interaction between warfarin and fluconazole, this antifungal was switched to Caspofungin 70mg IV as first day loading dose, followed by 50mg/day IV. During that week, the patient did not present fever again, was hemodynamically stable but her new set of blood cultures yielded C. parapsilosis once again on the tenth day of Caspofungin therapy. A second TEE was performed searching for fungal vegetations that could explain the persistent fungemia despite the prolonged antifungal therapy, but once again it was unremarkable. Caspofungin dose was increased to 150mg/day IV (invasive candidiasis dose according to the 2016 IDSA guidelines) for 1 week. Control blood cultures repeated daily from day 1 of the high Caspofungin dose were sterile and patient did not develop fever again.
She was finally discharged on oral Fluconazole 14 days after her last sterile blood culture, asymptomatic and hemodynamically stable. The patient was scheduled for follow-up in the infectious diseases clinic
DISCUSSION
The frequency of endocarditis due to C. parapsilosis has increased in recent years, this due to its ability to form biofilms which also explains its high mortality1, and according to the 2023 Duke ISCVIV Criteria, Candida spp. is now considered a "typical" infective endocarditis (IE) pathogen in the setting of intracardiac prosthetic material6.
For a successful outcome, an accurate diagnosis of IE is necessary to provide adequate treatment. In this case, the patient had two positive C. parapsilosis blood cultures which constitute a major criterion plus three minor criteria for IE: previous history of prosthetic valve, previous valve repair and fever. This confirmed the diagnosis.
On the other hand, despite having several echocardiographic procedures including TEE (which has high specificity and sensitivity especially in patients with prosthetic valves)7, no vegetations were identified. This could be explained by the fact that fungal vegetations have a higher incidence of embolic events compared to bacterial endocarditis, and these may had detached before TEE was performed8. In addition, it has been described that TEE could not confirm or exclude an IE diagnosis in challenging clinical situations such as patients with intracardiac implants6. In those scenarios, other diagnostic techniques such as cardiac computed tomography (CCT) and/or positron emission computed tomography (PET/CT) should be used in order to improve the identification of IE especially of prosthetic origin 6. In fact, CCT in combination with TEE has higher sensitivity for the detection of both valvular and paravalvular lesions, while PET/CT is preferred to diagnose PVE over echocardiography. Both imaging techniques are included in the 2023 Duke-ISCVID IE Criteria9.
Regarding clinical manifestations, common infectious endocarditis signs like heart murmurs may be absent in fungal endocarditis, however Candida endocarditis has certain characteristics such as loss of visual acuity or cutaneous nodules. Likewise, the vegetations are large and have a greater tendency to embolize7. Also, Garzoni in his review of the literature regarding C. parapsilosis endocarditis, found that 43.8% of 72 cases presented cerebral thromboembolic events, and he also mentioned that fever and embolic events should raise the suspicion of infective endocarditis, specially of fungal origin9. In addition, Candida spp is described in the literature as a cause of cardioembolic strokes and Lipton et al mentioned in their study that when Candida endocarditis is suspected, the rate of spread to the central nervous system can reach up to 80%10.
The definitive treatment for Candida endocarditis is the combination of antifungal therapy and surgery according to the 2016 IDSA candidiasis guidelines and the 2015 American Heart Association infective endocarditis guidelines11-13. However, it was suggested through a meta-analysis of Candida endocarditis, that combined antifungal therapy versus medical-surgical therapy have similar success rate in the specific case of C. parapsilosis14). The ESCAPE study concluded that the induction treatment should be based on Liposomal - Amphotericin followed by fluconazole maintenance therapy to prevent relapses15). However, other authors consider high dose echinocandins as the preferred options for initial therapy given their better tolerability and exceptional fungicidal activity in the presence of biofilms; this may be an exception for C. parapsilosis as it is sometimes resistant to echinocandins8,12,16,17).
On the physical exam, the patient did not show murmurs and four echocardiograms were unremarkable making the diagnosis challenging. However it is known that in patients with preexisting valvular disease, the detection of vegetations even by TEE can be suboptimal17. In fact, Hershman-Sarafov described in his retrospective analysis of IE that 44% of the 538 cases were echocardiogram-negative and mostly occurred on damaged valves and elderly patients18.
Our patient received a long course of IV fluconazole without a good clinical and microbiological response despite fluconazole susceptibility (MIC: 0.12). She started on IV caspofungin, however the blood cultures continued to grow C. parapsilosis until the dose was increased to 150mg/day, which is the dose recommended for invasive candidiasis11. This supports the idea that the patient had an invasive candidiasis (C. parapsilosis endocarditis and fungemia) since the specific caspofungin dose for this clinical presentation of candida infection led into a good outcome.
We must conclude that C. parapsilosis endocarditis incidence is rising and must be suspected in patients with risk factors such as prosthetic heart valves or valve disease, injection drug use history, indwelling central venous catheters, cancer chemotherapy, prior bacterial endocarditis or low birthweight neonates7,19-21. Antifungal susceptibility testing should always be performed and negative echocardiographic examinations, including TEE do not rule out infective endocarditis especially in the presence of a prosthetic valve.