Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Sociedad Química del Perú
Print version ISSN 1810-634X
Rev. Soc. Quím. Perú vol.80 no.3 Lima July/Set. 2014
TRABAJOS ORIGINALES
Phytochemical study of Echinopsis Peruviana
Estudio fitoquímico de Echinopsis Peruviana
Pedro A. Baldera-Aguayo1; Víctor M. Reyna Pinedo1
1 Universidad Nacional de Ingeniería, Escuela Profesional de Química, Facultad de Ciencias. Av. Túpac Amaru 210 Rímac, Lima 25, Lima, Perú. vreyna@uni.edu.pe
ABSTRACT
The San Pedro Macho cacti (Echinopsis peruviana or also known as Trichocereus peruviana) owes its name and importance, in part, to its taxonomical and alkaloid composition similarity to the well-known San Pedro Hembra cacti (Echinopsis pachanoi) or commonly referred as San Pedro, which is the most important species among the plants used in Traditional Medicine (TM) in Northern Perú. This phytochemical report of an Echinopsis peruviana species cultivated in Perú involves the qualitative analysis of its secondary metabolites, the quantification of the total alkaloid content and the isolation of the alkaloid mescaline, as its sulphate dihydrate salt, of the chlorenchyma in the green outer cortex of the stems of E. peruviana. ESI-MS; 1H, 13C NMR; IR; UV; elemental analysis and TLC techniques were used to characterize this compound.
Key words: Echinopsis peruviana, San Pedro Macho, mescaline, phytochemical analysis, alkaloid content and phenylethylamine alkaloids.
RESUMEN
El cactus San Pedro Macho (Echinopsis peruviana o también conocido como Trichocereus peruviana) debe en parte su nombre y su importancia a su similaridad, tanto taxonómica como en su contenido de alcaloides, con el muy conocido cactus San Pedro Hembra (Echinopsis pachanoi) o comúnmente denominado San Pedro, el cual es la especie más importante entre las plantas empleadas en Medicina Tradicional (MT) en el norte del Perú. Este trabajo fitoquímico de la especie Echinopsis peruviana cultivada en Perú comprende el análisis cualitativo de sus metabolitos secundarios, la cuantificación total de alcaloides y el aislamiento del alcaloide mescalina como su sal, el sulfato dihidratado, a partir de la corteza de los tallos del cactus E. peruviana. Las técnicas ESI-MS; RMN 1H, 13C; IR; UV; análisis porcentual de elementos y Cromatografía en Capa Fina (CCF) fueron empleadas para caracterizar al compuesto aislado.
Palabras clave: Echinopsis peruviana, San Pedro Macho, mescalina, análisis fitoquímicos, contenido de alcaloides y alcaloides feniletilamínicos.
INTRODUCTION
Peru is ranked in the list of the twelve most mega-diverse countries in the world. Due to its unique geographical conditions, Peru is home to a diversity of ecosystems holding at least 25000 species of plants (approximately 10% of the worldwide species of flora), of which 5354 are endemic and hosted in its numerous ecological environments1. Among which, 252 plants correspond to different endemic species of cactus2.
Furthermore, Peru is an outstanding example with a millennia old tradition of healers using the rich flora, and particularly in northern Peru at the departments of Lambayeque, La Libertad and Piura, in which Traditional Medicine (TM) practices are part of everyday life such as the Mesas (healing altars) with San Pedro, which is one of the most well-known therapeutic process in the northern area3-11.
Regarding the alkaloid composition in the Echinopsis peruviana and pachanoi species, there is no substantial evidence to conclude that the alkaloid content in San Pedro Macho (E. peruviana) is higher compared to that of San Pedro Hembra (E. pachanoi), mainly because the existing studies, so far, have employed species collected from different sources7, 12-17. As a matter of fact in the field of phytochemistry and stressed out by Trout (2005)17, the sources of significant differences in the total alkaloid content as well as in the amount of individual alkaloids present in the cactus rely on the taxonomy of species, growing and harvesting conditions, to mention a few.
In contrast to the San Pedro Hembra cactus, which is widely employed in TM, San Pedro Macho is overlooked for therapeutic purposes. The reason of this fact lies on the ease collection and manipulation of Echinopsis pachanoi because is a cultivated species, while, the E. peruviana cacti is a wild, non-cultivated species, whose long, thick and needle-lick spines difficult its harvesting and handling (Victor M. Reyna Pinedo's field observations).
Our work has focused on the phytochemical study of Echinopsis peruviana collected from the Cañete river basin in Lima-Peru, a region that has not been fully explored until 2006, when Ostolaza et al. (2007)18 reported for the first time the cacti and other succulent species occurring in this area. More important, results from this work stressed out that E. peruviana should be considered as EN (Endangered) species according to the Red List of the International Union for the Conservation of Nature (IUCN)18.
Our phytochemical investigation comprises the qualitative analysis by phytochemical screening and quantitative analysis of the total alkaloid content by potentiometric titration. Furthermore, the alkaloid mescaline was isolated as its sulfate dihydrate salt, and its structure was confirmed by different techniques such as ESI-MS; 1H, 13C NMR; IR; UV; elemental analysis and TLC19.
EXPERIMENTAL19
Instruments
Melting points were obtained on a IA9000 Digital Melting Point apparatus. Silica gel 60G F254 Glassplates were used for analytical TLC, and compounds were visualized under UV light with a Vilber Lourmat lamp and subsequently detected after spraying with fluorescamine reagent. IR spectra were measured on a Shimadzu FTIR instrument as KBr discs. UV measurements were obtained on a Shimadzu UV–Visible 2450 spectrophotometer. NMR spectra were collected on a Bruker Avance 500 MHz instrument for solutions in DMSO-d6 for 1H and D O for 13C and DEPT experiments. ESI–MS was performed on an Agilent 6890N Ser. II apparatus, fitted with a fused silica HP–1 capillary column (30 m x 0,25 mm i.d.; 0,25 m film thickness), coupled to an Agilent Mass Selective Detector MSD 5973; ionization energy voltage 70 eV; electron multiplier voltage energy 2000 V. Mass spectra were scanned in the range 50 – 800 amu, scan time 5 scans/s. Elemental analysis for C, H, N, S (combustion) and O (pyrolysis) was performed on an Elementar Analyzer MICRO cube instrument. A pH-meter PL–700 PV was employed for the potentiometric titration analysis.
Plant material
The stems of Echinopsis peruviana were collected in Huancayo (Reserva Paisajistica Nor Yauyos–Cochas), Yauyos, Lima, Peru, on August 8th 2008. The plant was identified by Dr. Carlos Ostolaza Nano.
Phytochemical analysis
Screening for major constituents: primary and secondary amines, free phenolics, tannins, flavonoids, triterpens, steroids, quinones, leucoantocianidines, catechins, saponins and alkaloids were carried on according standard phytochemical screening proposed by Rondina and Coussio (1969)20.
Total alkaloid content assay
About 6 g of exactly weighed powdered plant material (chlorenchyma from the green outer cortex of the stems of E. peruviana) was dispersed in a mixture of 10 mL of ammonium hydroxide and 30 mL of bidistilled water, then the solution was washed with four 40 mL portions of the mixture chloroform and peroxide–free ether (1:3) until no positive reaction was observed with Dragendorff and Mayer reagent20. The combined organic layers were concentrated to about 50 mL by distilling on a rotary evaporator and the resulting liquid was transferred to a separating funnel, rinsing with peroxide–free ether. A volume of peroxide–free ether equal to 2,1 times the volume of the combined organic layers was added. The resulting solution was washed with five 25 mL portions of H2SO4 0,5 N until the alkaloids were completely extracted. The acid layers were made alkaline with ammonium hydroxide 26% until pH 12. The basic aqueous phase was extracted with 4 x 30 mL chloroform until the organic solvent displayed no reaction with Dragendorff and Mayer reagents, then the chloroform extracts were dried over anhydrous sodium sulphate and allowed to stand for 30 min with occasional shaking and filtered. The sodium sulphate remnant were washed four times with chloroform, each of 10 mL. The combined chloroform extracts were evaporated to dryness on a rotary evaporator to afford the alkaloid extract. The extract obtained was heated in an oven at 100 – 105 °C for 15 min. Then, the extract was dissolved in a few milliliters of chloroform, evaporated to dryness on a rotary evaporator and heated again in an oven at 100 – 105 °C for 15 min. Finally, the alkaloid extract was dissolved in 5 mL of H2SO4 0,02 N solution on a water bath (50 – 60 °C) in order to remove the chloroform by evaporation. The acidic solution was potentiometrically titrated with NaOH 0,02 N. The procedure was performed in a series of four parallel experiments.
Isolation of mescaline alkaloid
Dried and powdered chlorenchyma from the green outer cortex of the stems of E. peruviana (100 g) were extracted twice with boiling H2SO4 0,5 N (1 L) and once with distilled water (1 L, 10% w/v). After the extract filtration, a spontaneous emulsion at room temperature was yielded which was separated by centrifugation. The pellet was discarded and the supernatant liquid (3 L) was alkalinized to pH 12 with 6 N sodium hydroxide (the pKa of the protonated amino group of mescaline being 9,5) and extracted with chloroform (2 x 300 mL). The aqueous layer was discarded, and the chloroform was evaporated to dryness. The extract (4,5 g) was redissolved in cooled 95% ethanol (5 °C) and a solution of H2SO4 (1% w/w) was added at a moderated rate. The white precipitated was washed several times with 95% ethanol, yield 137,77 mg (0,14%). The salt, after two recrystallizations from 95% ethanol melted at 184 °C.
RESULTS AND DISCUSSION
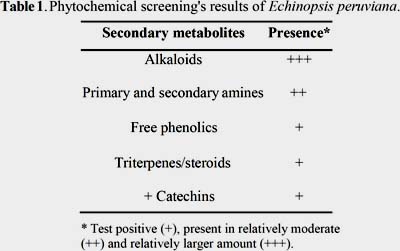
The qualitative phytochemical analysis carried out in Echinopsis peruviana using the standard phytochemical screening test proposed by Rondina and Coussio (1969)20 revealed the presence of alkaloids, primary and secondary amines, free phenolics, triterpens, steroids and catechins. Among these, alkaloid compounds were found to be the most abundant components (table 1).
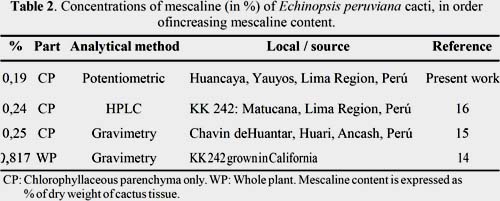
The quantification of the total alkaloid content was conducted using the standard test of the European Pharmacopoeia (2005)21. The results from four parallel experiments were reported as their average percentage value: 0,19 ± 0,03%. Our result is in good agreement with the values reported by Ogunbodede et al. (2010)16 and Cjuno et al. (2009)15 (table 2). Moreover, our result supports the notion that the mescaline concentration in E. peruviana is lower compared to that of E. pachanoi, as previously suggested by Ogunbodede et al.(2010)16
Djerassi et al.(1955)12 did not detected mescaline from E. peruviana wild harvested in Peru (they did not mention where the material originally was collected from) because their procedure was flawed for detecting only the alkaloid that were ether-soluble. Likewise, Agurell (1969)13 did not report the presence of mescaline, but he did confirm the occurrence of tyramine and 3-methoxytyramine in a sample of E. peruviana from an European commercial nursery stock in The Netherlands or Sweden.
The solid-liquid extraction and liquid-liquid separation of a dried and powdered sample of chlorenchyma from the green outer cortex of the stems of E. peruviana yielded a brown fraction, from which mescaline was further isolated as its sulfate dihydrate salt (figure 1).
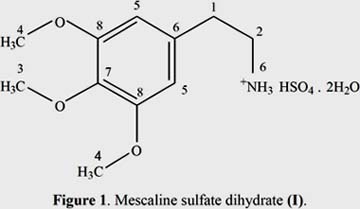
Compound (I) was isolated as a white amorphous solid (MP: 184ºC) with a molecular formula of C22H40N2O12S, which was established by elemental analysis (C 47,31; H 7,07; N 5,08; O 34,77; S 5,73). The chromatography behavior of compound (I) was examined on TLC silica gel 60 F254 (pore 0,74 – 0,84 ml/g) in two mixtures of solvents: i. Ethyl acetate – MeOH (8,5:1,0) and ii. Diethyl ether – acetic acid glacial (19:1). The Rf values are 0,66 and 0,54, respectively.
In the ESI-MS of (I) we note the 211 peak of the molecular ion, as well as the characteristic mass peaks 182, 167 and 151 of mescaline7, 22-26 (figure 2).
The 1H NMR spectrum of (I) in DMSO-d (figure 3) showed signals at ä 6,52 (2H, s, J = 14,1 Hz, H-6) corresponding to a para tetrasubstituted aromatic ring and protons of three methoxy groups attached to aromatic ring at 3,76 (6H, s, H-5) and 3,62 (3H, s, H-4). A singlet was identified at ä 3,32 (2H, s) partially overlapping with the residual HDO signal, and was assigned to H-3. The signals at ä 2,65 - 2,68 (2H, t, J = 7,4 Hz) and ä 2,88 - 2,91 (2H, t, J = 7,4 Hz) correspond to H-1 and H-2, respectively (table 3).
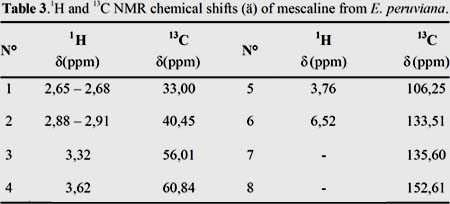
In the 13C NMR spectrum in D O (figure 4), resonances of the tetrasubstituted benzene ring at ä 152,61 (C-8), 135,60 (C-7), 133,51 (C-6), 106,25 (CH, C-5) were observed, as well as three methoxy groups at ä 60,84 (C-4) and 56,01 (C-3). The signals at ä 33,00 and 40,45 correspond to the methylene groups at C-1 and C-2, respectively (table 3).
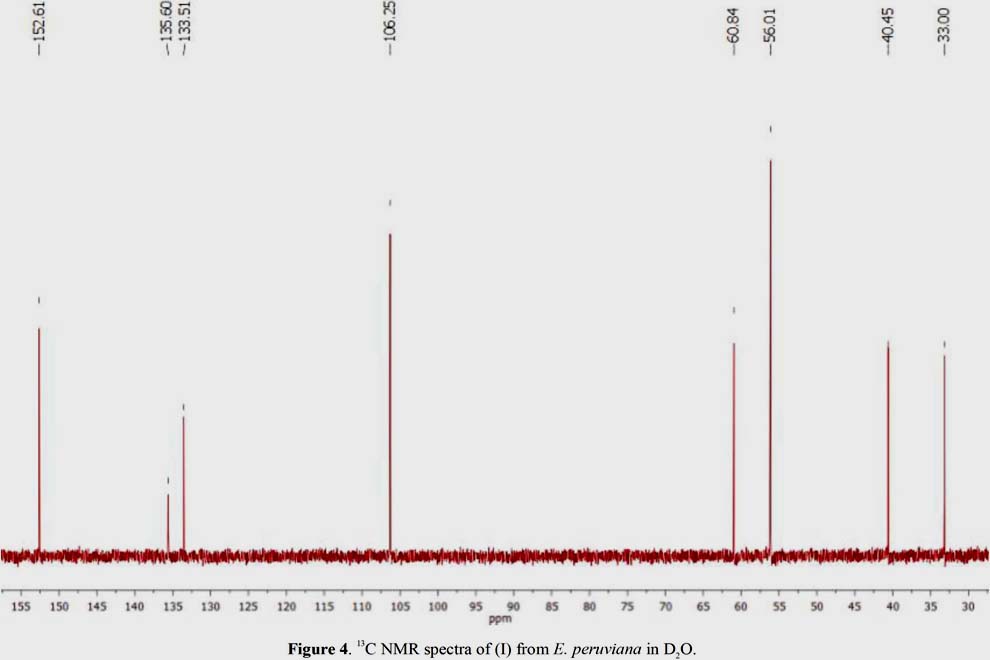
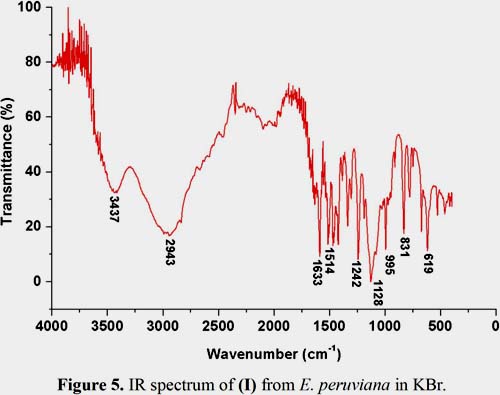
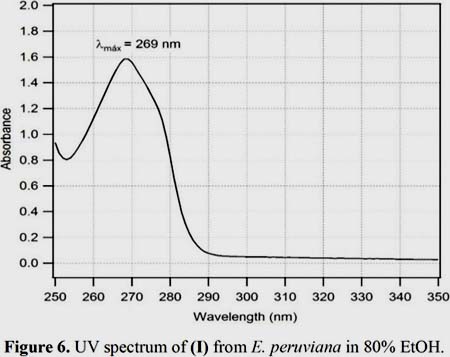
Figure 5 shows that the major peaks in the IR spectrum (KBr) of (I) occur at the following wavenumbers (cm-1): 3437 N-H stretch (-+NH3), 2943 C-H asymmetrical stretch, 1633 N-H bend (-+NH3), 1514 CH3 scissoring, 1242 S-O stretch (SO42-) 1128 C-O symmetrical and asymmetrical stretch (=C-O-C), 995, 831 and 619 correspond to C-H bend in the aromatic ring. The UV spectrum of (I) in 80% EtOH (figure 6) shows a maximum absorption (ëmax) at 269 nm (log å = 5,95). ESI-MS, NMR, IR and UV analysis are in excellent agreement when compared with literature data7, 22-26.
CONCLUSIONS
Mescaline was isolated as its sulphate dihydrate salt from the stems of San Pedro Macho (Echinopsis peruviana) and it was characterized by ESI-MS, 1H, 13C NMR, IR, UV, elemental analysis and TLC techniques. Moreover, the phytochemical screening analysis of the plant showed the presence of alkaloids (+++), primary and secondary amines (++), free phenolics (+), triterpenes and/or steroids (+) and catechins (+). Finally, the quantification of the total alkaloid content from the stems of E. peruviana, 0,19 ± 0,03%, is in good agreement with other values previously reported.
ACKNOWLEDGEMENTS
The authors are indebted to Dr. Michael Sauvain (IRD) and Dr. Carlos Ostolaza Nano for the collection and identification of the plant material, respectively. We also thank Dr. Eric Paulson and Dr. Xiaoling Wu (CBIC, Yale, USA) for ESI-MS and NMR spectra, to Dr. Bernard Delpech (ICSN–CNRS, France) for elemental analysis and to Lic. Jorge Muñante F. for IR spectra (EMPESA, Perú).
REFERENCES
1. Brack Egg A. Diccionario enciclopédico de plantas útiles del Perú. Cusco: Centro de Estudios Regionales andinos "Bartolomé de Las Casas"; 1999.
2. Ostolaza Nano CA. 101 Cactus del Perú, Lima: Ministerio del Ambiente; 2011.
3. Camino L. Cerros, plantas y lagunas poderosas, la medicina tradicional al norte del Perú. Lima: Lluvia Editores; 1992.
4. Polia Meconi M. Las lagunas de los encantos: Medicina tradicional andina del Perú septentrional. Piura: Central Peruana de Servicios (CEPESER); 1989.
5. Polia M. Despierta, remedio, cuenta: adivinos y médicos del Ande. Lima: Pontifícia Universidad Católica del Perú, Fondo Editorial; 1996.
6. De Feo V. Medicinal and magical plants in the northern Peruvian Andes. Milano: Fitoterapia; 1992; 63 (5): 417-440.
7. Joralemon D, Sharon D. Sorcery and shamanism: curanderos and clients in northern Peru. Salt Lake City: University of Utah Press; 1993.
8. Reyna Pinedo VM, Flores Garcés J. El uso del "San Pedro" (Echinopsis pachanoi) en medicina tradicional peruana. Quepo 2001; 15: 28-37.
9. Bussmann RW, Sharon D. Traditional medicinal plant usein Northern Peru: tracking twothousand years of healing culture. J Ethnobiol Ethnomed 2006; 2: 47-65.
10. Reyna Pinedo VM, Carbajal FM, Carbajal RJ. Estudio Etnomedicinal de las Mesas con San Pedro. Verificación de casos de curación. Cult. Drog. 2009; 14:79-88.
11. Reyna Pinedo VM, Carbajal FM, Carbajal Rodríguez J. Estudio Etnomedicinal de las Mesas con San Pedro II. Mesas de Don Marco Carbajal F. Año 2009. Cult. Drog. 2010; 15: 29-46.
12. Djerassi C, Liu LH, Farkas E, Lippman AE, Lemin AJ, Geller LE, McDonald RN, Taylor BJ. Terpenoids. XI.1 Investigation of Nine Cactus Species. Isolation of Two New Triterpenes, Stellatogenin and Machaeric Acid2. J Am Chem Soc 1955; 77 (5): 1200- 1203.
13. Agurell S. Cactaceae alkaloids. I. Lloydia 1969; 32 (2): 206-216.
14. Pardanani JH, McLaughlin JL, Kondrat RW, Cooks RG. Cactus alkaloids. XXXVI. Mescaline and related compounds from Trichocereus peruvianus. Lloydia 1977; 40 (6): 585-590.
15. Cjuno M, Choquenaira J, Quispe P, Serrano C, Tomaylla C. El género Trichocereus, ecología y contenido mescalínico. Quepo 2009; 23: 38-45.
16. Ogunbodede O, McCombs D, Trout K, Daley P, Terry M. New mescaline concentrations from 14 taxa/cultivars of Echinopsis spp. (Cactaceae) ("San Pedro") and their relevance to shamanic practice. J Ethnopharmacol 2010; 131: 356-362.
17. Trout K. Trout's Notes on San Pedro & Related Trichocereus Species. Austin: Better Days Publishing; 2005.
18. Ostolaza C, Ceroni A, Zapata J, Cortéz J, Salinas L, García E. Cacti of the Cañete river basin, Lima, Peru: a research and conservation study. Cactus World 2007; 25: 215-226.
19. Baldera-Aguayo, PA. Estudio químico del cactus San Pedro Macho (Echinopsis peruviana) [Tesis de Licenciatura en Química]. Lima, Perú: Universidad Nacional de Ingeniería – Facultad de Ciencias; 2014.
20. Rondina RV, Coussio JD. Estudio fitoquímico de plantas medicinales argentinas. Revista de Investigaciones Agropecuarias, INTA, Serien 2, Biología y Producción Vegetal, Buenos Aires, Argentina, 1969; 6 (22): 351-366.
21. Council of Europe. European Pharmacopoeia 5th ed. Strasbourg: Council of Europe; 2005, p. 1060-1061.
22. Mills III T, Roberson JC, Matchett CC, Simon MJ, Burns MD, Ollis Jr RJ. Instrumental Data for Drug Analysis. Florida: CRC Press; 2006, vol. 6, p. 1862-1863.
23. Henry JL, Epley J, Rohrig TP. The analysis and distribution of mescaline in postmortem tissues. J Anal Toxicol 2003; 27: 381-382.
24. Fucci N, Chiarotti M. Mescaline in multi-coloured statuettes. Forensic Sci Int 1996; 82 (2): 165-169.
25. Becker H. Inhaltsstoffe der Kaktee Lophophora williamsii. Pharm Unserer Zeit 1985; 14 (5): 129-137.
26. Bailey K, Legault D. 13C NMR spectra and structure of mono-di-and trimethoxyphenylethylamines and amphetamines. Org Magn Resonance 1983; 21 (6): 391-396.
Recibido el 26-08-2014
Aprobado el 02-09-2014
















