Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Sociedad Química del Perú
Print version ISSN 1810-634X
Rev. Soc. Quím. Perú vol.83 no.1 Lima Jan./Mar. 2017
TRABAJOS ORIGINALES
Release of anthocyanins from chitosan films cross-linked with sodium tripolyphosphate
Liberación de antocianinas a partir de películas de quitosano entrecruzadas con tripolifosfato de sodio
Max J. Carlos-Salazar*1, Ana C. Valderrama-Negrón1
1 Laboratorio de Biopolímeros y Metalofármacos, LIBIPMET, Facultad de Ciencias, Universidad Nacional de Ingeniería. Av. Túpac Amarú 210, Rímac Lima 25 – 15333. Lima, Perú. mcarloss@uni.edu.pe
ABSTRACT
Chitosan and depolymerized chitosan films cross-linked with sodium tripolyphosphate were prepared and loaded with anthocyanin extracted from purple corn. The effect of sodium tripolyphosphate concentration, cross-linking time, thickness and molar mass of chitosan on the delivery of anthocyanin were studied. The results showed a lower percentage of available anthocyanin (from 29 % to 10 %) in the films more cross-linked, it staying the remaining anthocyanin in the film. This percentage was influenced by the other factors mentioned above. The release kinetics has been studied to elucidate the drug release mechanism. All films was controlled by Fick’s law when fitted to the Higuchi model. For the determination of the diffusion exponent, the results were fitted to the Korsmeyer-Peppas model. The films showed different mechanisms of release, including a Fickian and a non-Fickian diffusion process.
Key words: Chitosan, sodium tripolyphosphate, films, drug release.
RESUMEN
Fueron preparadas películas de quitosano y quitosano despolimerizado entrecruzados con tripolifosfato de sodio y cargados con antocianinas extraídas de maíz morado. Fue estudiado el efecto de la concentración de tripolifosfato de sodio, tiempo de reticulación, espesor y masa molar de quitosano en la liberación de antocianinas. Los resultados mostraron un bajo porcentaje de antocianinas disponibles (de 29% a 10%) en las matrices más entrecruzadas, manteniendo el resto en las películas. Este porcentaje fue influenciado por los otros factores anteriormente mencionados. La cinética de liberación ha sido estudiada para dilucidar el mecanismo de liberación de los fármacos. Todas las películas fueron controladas por la ley de Fick cuando se ajustaron al modelo de Higuchi. Para la determinación del exponente de difusión, los resultados fueron ajustados al modelo de Korsmeyer Peppas. Las películas mostraron diferentes mecanismos de liberación, incluyendo procesos de difusión Fickianos y no Fickianos.
Palabras clave: Quitosano, tripolifosfato de sodio, películas, liberación de fármacos.
INTRODUCTION
In recent years, the formulation of drug delivery systems has been a topic of great interest to the pharmaceutical industry. Several new systems are being investigated to develop improved devices in comparison with the conventional forms of drug dosage1. This could be done by modifying the velocity, the time and the location of drug release. This would eventually make it possible to achieve the desired therapeutic drug level at the site of interest and maintain it for a long time.
Currently, a large amount of polymers are used for the manufacture of release systems. These systems use synthetic or natural polymers as drugs carriers, while implantable biomaterials mainly serve as physical support devices. For many applications, biomaterials have to comply with an extensive set of requirements to ensure their safety. In this regard, natural polymers are of particular interest due to their nontoxic, biocompatible, biodegradable and hydrophilic nature. Among the natural polymers used for drug release, chitosan (CS) has been widely studied due to its ease of chemical modification and its favorable biological properties2. Chitosan is one of the most important of those polymers and is manufactured from the chitin obtained from many marine crustaceans, such as shrimps and crabs3.
Chitin (2-acetamide-2-deoxy-β-D-glucose) undertakes deacetylation through β (1→4) linkage, so as to obtain chitosan, a copolymer of 2-amine-2-deoxy-β-D-glucose and 2-acetamide-2-deoxy-β-D-glucose4. Chitosan is used for the preparation of hydrogels, microcapsules, microspheres, nanoparticles, fibers and films; most of these products are for biomedical applications where biocompatibility is essential. Chitosan may be cross-linked with different reagents such as polyethylenimine, polyvinyl alcohol, polyethylene glycol, glutaraldehyde, etc. Many chitosan matrices are obtained by treatment with multivalent anions, such as glycerophosphate, oxalic acid, as well as tripolyphosphate. The formation of a chitosan/tripolyphosphate complex has gathered a lot of interest since it is a very simple process, and the polyanion can form hydrogels with chitosan through ionic interaction. Furthermore, sodium tripolyphosphate is a compound classified as Generally Recognized As Safe (GRAS) by the Foods and Drugs Administration5.
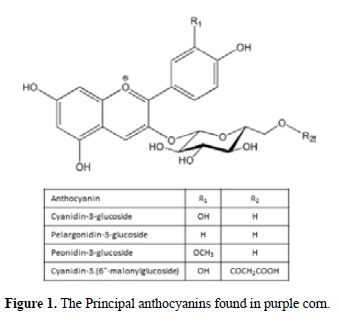
Anthocyanins are natural dyes which have raised a growing interest due to their wide range of colors. So far, more than 635 natural anthocyanins have been identified; this versatile group is responsible for the intense blue, purple and red colors of many fruits, vegetable and flowers. It has been suggested that anthocyanins possess anti-inflammatory, anti- carcinogenic and diabetes alleviation properties, and that they can be used for the prevention of cardiovascular disease and obesity control, all these characteristics being more or less associated with their powerful antioxidant properties6. Purple corn is an important source of anthocyanin and has been cultivated for centuries in the Andean Region of Latin America7. The anthocyanins identified in purple corn cobs and seeds include cyanidin-3-glucoside, pelargonidin-3-glucoside, peonidin-3-glucoside and their respective malonated counterparts.
This work shows the release of purple corn anthocyanins from chitosan films cross-linked with tripolyphosphate, as well as the determination of the delivery mechanism and its compliance with Fick’s law.
MATERIALS AND METHODS
Materials
Chitosan (CS) was purchased from Sigma-Aldrich (Ireland) (deacetylation degree 80.16 %, Mw = 554.22 kDa). It was depolymerized and characterized (deacetylation degree 81.78%, Mw = 133.37 kDa), so as to obtain depolymerized chitosan (CSD)8. The deacetylation degree was determinated by conductimetric titration and the molar mass was obtained by viscosimetry9. Purple corn (Zea mays L.) anthocyanins under the form of a powder extract, was donated by Globenatural International S. A. (Peru). Sodium tripolyphosphate (TPP) was purchased from Sigma-Aldrich (USA); all other reagents were of analytical grade.
The depolymerization of Chitosan
The depolymerized chitosan was obtained for oxidative degradation using sodium nitrite. It was prepared chitosan solution 1% (w/v) in acetic ácid 1% (w/v) and NaNO 0.1M was added such the molar relation in chitosan and sodium nitrite was 0.01. The solution was stirring for 3 h and added NaOH until pH 8. Subsequently was washed with water, centrifugated and dried at 45 °C to constant weight8.
Film preparation
The chitosan/TPP films loaded with anthocyanin were obtained by the solvent evaporation technique. The anthocyanin powder (0,1 or 0,2 g) was mixed with a chitosan solution (1%, w/v) in acetic acid (1%, w/v) and was stirred for 4h. The solution was then poured on an acrylic surface (10 x 10 cm) and dried at 45°C for 24h. The resulting films were immersed in 100mL of TPP aqueous solutions of varying concentrations. The cross-linked films were then washed with distilled water and dried at 60°C for 30 min. The variables studied were: volume of chitosan solution (25 or 50 mL); concentration of TPP (0 to 5% w/v); cross-linking time (0 to 2h). Therefore was proposed a nomenclature for each film, "A" for films prepared with chitosan and "B" for films prepared with depolymerized chitosan + a number according to the first colum of the table 1. The films was prepared with nomenclature A1, A2, A3, A4, A5, A6, B1, B2, B3, B4, B5 and B6. The amount of anthocyanin lost during the cross-linking process was measured by UV-VIS absorption at 515,2nm9,10.
Anthocyn in in vitro release
The chitosan/TPP films loaded with anthocyanin were immersed in 50 mL of a phosphate buffered saline solution at pH 6,5, 37°C and stirred at 100rpm. For measurement purpose, 2mL of release medium were extracted at predetermined times and replaced with the same volume of fresh medium. The anthocyanin concentration was measured at 515,2nm using a UV-VIS Shimadzu UV-1800 spectrophotometer.
Anthocynin release kinetics
The results were analyzed to evaluate the fit of the drug release results with the Higuchi and Korsmeyer-Peppas models11,12. The Higuchi model is based on Fickâs law, using the equation:
Q=KHt1/2 (1)
where Q is the quantity of anthocyanin released and KH is the Higuchi diffusion constant. The Korsmeyer-Peppas model is described by:
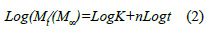
where Mt is the quantity of anthocyanin released after time t, Mâ is the quantity released after an infinite time, K is the system constant and n is the diffusion exponent. The value of n has a range of 0,5 to 1,0, with n = 0,5 corresponding to Fickâs diffusion process.
RESULTS AND DISCUSSIONS
Before undertaking the present work, a test of anthocyanin degradation was carried out in the same conditions as the drug release experiment. Results are not presented here, but it was found that the effect of time, temperature, light and conditions of delivery on the degradation of anthocyanin was insignificant.
Effect of tripolyphosphate concentration
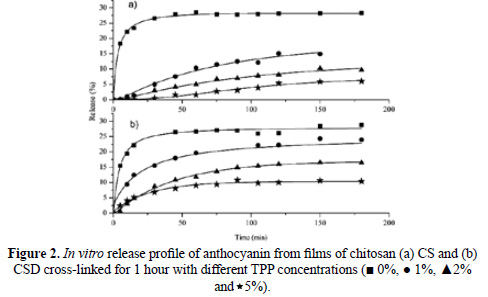
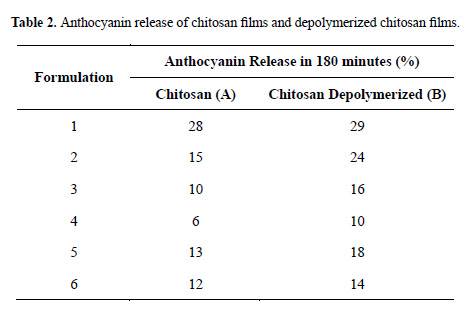
In Figure 2 is shown the percentage of anthocyanin released from chitosan films âAâ and âBâ cross-linked with various concentrations of TPP, for up to 180 minutes of delivery. It can be seen that an increase in the TPP cross-linking concentration produces a decrease in the amount of released anthocyanin. The release percentages obtained for CS/TPP films cross-linked with 0%, 1%, 2% and 5% TPP at infinite time were 28%, 15%, 10% and 6% respectively. In the case of CSD/TPP films cross-linked under the same conditions, the release percentages after 180 minutes were 29%, 24%, 16% and 10% respectively. In both cases, the effect was similar. An increase in the tripolyphosphate concentration provoked an increase in the crosslinking of the film, due to an increased interaction between the oxygen of the polyanion and the hydrogen of the protonated amine in the chitosan through hydrogen bonding. The resulting cross-linking slows down the anthocyanin release process
Effect of cross-linking time
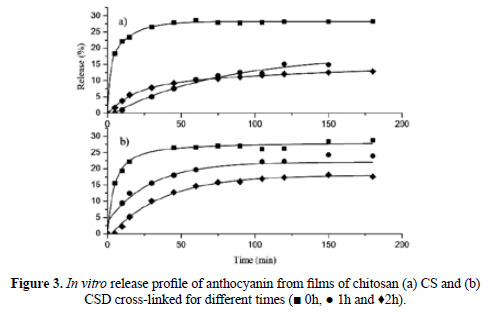
In Figure 3 is shown the delivery of anthocyanin from chitosan films cross-linked with 1% (w/v) TPP for different times. It can be observed that the smallest amount of release anthocyanin corresponds to the longest crosslinking time (2 h). After 180 minutes of delivery, the release percentages for chitosan films cross-linked with 1% TPP during 0, 1 and 2 h were 28%, 15% and 13% respectively, while for depolymerized chitosan films in the same condition, the release percentages were 29%, 24% and 18%. For chitosan films, the effect of cross-linking time is not significant, as the percentages of anthocyanin released from films cross-linked for 1h and 2h were similar.
Effect of thickness
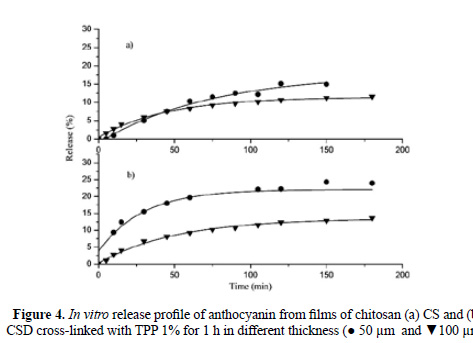
Films were prepared with two different volumes of solution (25 and 50 mL) in order to obtain different thicknesses, approximately 50 and 100 μm respectively. As can be observed in Figure 4, the thicker films released anthocyanin more slowly. After 180 minutes, the chitosan films of 50 and 100 µm had released 15% and 12% of the anthocyanin, respectively. The depolymerized chitosan films of 50 and 100 µm released 24% and 14% of the anthocyanin, respectively. This is due to the fact that the anthocyanin has to travel a greater distance to cross the polymer matrix.
Effect of molar mass
In Figures 2, 3 and 4 allow a comparison of the anthocyanin released by films with the same preparation, but differing in molar mass: 554.22kDa for chitosan films (CS) and 133,37kDa for depolymerized chitosan films (CSD). Table 2 shows that the percentage of anthocyanin released by the chitosan film is smaller than that released by the depolymerized chitosan film. This phenomenon is due to the reduced mobility of the high-weight chitosan molecules, which impedes the movement of the anthocyanin molecules, and therefore diminishes their release, as compared to the low-weight chitosan molecules.
Another important observation related to the delivery of anthocyanin is that the final percentage of release is low; the maximum reached was 29% for chitosan film B1. Most of the anthocyanin is retained because of its packaging in the film. Due to the presence of hydroxyl groups in the anthocyanin structure (see Figure 1) and the Hydroxyl and amine groups in the chitosan, exists the possibility of the formation of the hydrogen bonds and electrostatic interaction. In previous research, it has been reported that chitosan can be used to extract anthocyanins from radish, the dominant effect in this absorption phenomena being electrostatic attraction, including hydrogen bonding and charge neutralization13,14.
A similar result has also been obtained in the case of riboflavin released from chitosan films cross-linked with tripolyphosphate and pyrophosphate, where the increase in electrostatic interaction between anions and chitosan influences film properties and drug release significantly10. In another study, the pH dependence of the development of chitosan- tripolyphosphate fibers by ionotropic gelation highlighted the interaction of chitosan and tripolyphosphate in this matrix15.
Kinetic behavior of anthocyanin release
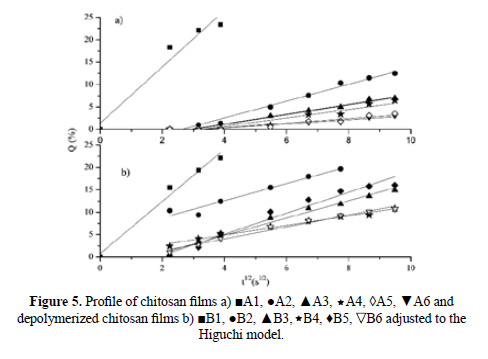
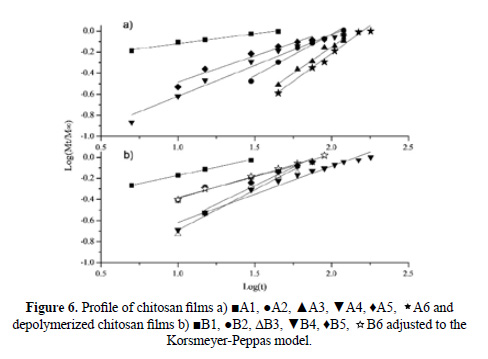
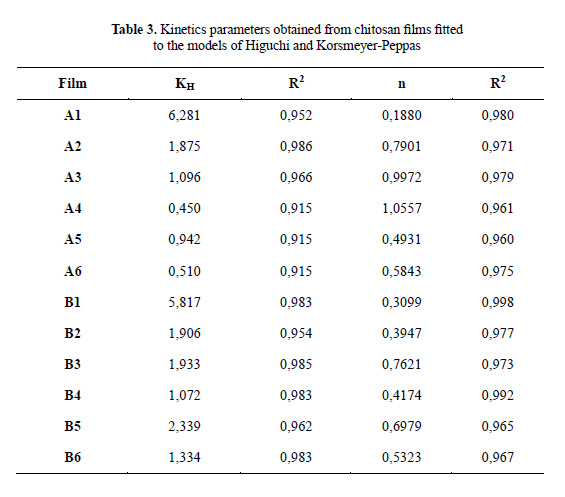
The results obtained for the release kinetics were compared to the Higuchi model. In the Figure 5 is shown the percentage of release Q vs t1/2, where the results for all the films can be fitted to straight lines with high correlation coefficients (R2 larger than 0.91). The delivery of anthocyanin can be described by a diffusion process based on Fick’s law. In order to determine the delivery mechanism, the data was also compared to the Korsmeyer-Peppas model (2). In Figure 6 is shown the Log (Mt/M∞) vs Log (t) plot obtained for chitosan and depolymerized chitosan films.
Table 3 shows the calculated parameters for both models. The films show different diffusion exponents: for A1, B1, B2 and B4 films, n < 0,5, corresponding to the slab release mechanism (non swellable matrix)16; for A5, n ≈ 0,5, the system in this case is controlled by Fick’s law. The A2, A3, A6, B3, B5 and B6 films show 0,5 < n < 1, presenting a case of anomalous diffusion (non-Fickian), controlled by processes of diffusion and relaxation of polymer chain. For the A4 film, n ≈ 1 and the process has been called transport case II12.
Similar results have been obtained previously for the release of potassium sorbate from chitosan films, where the release mechanism (Fickian or non-Fickian diffusion) depends upon the concentration of potassium sorbate with various behaviors and release mechanisms17. In the case of other polymer matrices, such as propranolol hydrochloride released from chitosan/ genipin/poly(N-vinyl-2-pyrrolidone) films, the diffusion exponent is different for each film prepared, indicating cases of non-Fickian diffusion and transport super-case II18.
Different chitosan-tripolyphosphate and depolymerized chitosan-tripolyphosphate films were prepared and loaded with anthocyanins. Variations in tripolyphosphate concentration, crosslinking time and thickness show that ionic cross-linking influences the release mechanisms of anthocyanin. Variations in the molar mass of chitosan show that more anthocyanin is released when the polymeric chain is smaller. The release mechanism controlling delivery was Fickian and non-Fickian diffusion. The percentage of anthocyanin released by the polymer matrix was less than 29% in all cases.
ACKNOWLEDGMENTS
The authors gratefully acknowledge financial help from LIBIPMET and Universidad Nacional de Ingeniería.
REFERENCES
1. Chowdary KP, Chaitanya ChK. Recent research on floting drug delivery systems-a review. JGTPS. 2014; 5(1): 1361-1373. [ Links ]
2. Sonia TA, Sharma CP. An overview of natural polymers for oral insulin delivery. Drug Discov Today. 2012; 17(13/14): 784-792. [ Links ]
3. Abreu FO, Cavalcante LG. Doudement PV. Castro AM. Nascimento AP. Propriedades e Características da Quitosana Obtida a Partir do Exoesqueleto de Caranquejo-Uçá Utilizando Radiação de Microondas. Polímeros. 2013; 23(5): 630-635. [ Links ]
4. Rianudo M. Chitin and chitosan: Properties and applications. Prog Polym Sci. 2006; 31(7): 603-632. [ Links ]
5. CFR, Code of Federal Regulations. Title 21, Volume 3 ̶ Food and Drugs. Sodium Tripolyphosphate. Washington DC. [access on February 10 of 2017]. Available in: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.1810. [ Links ]
6. He J, Giusti MM. Anthocyanins: Natural colorants with health-promoting properties. Annu Rev Food Sci Technol. 2010; 1(1): 163-186. [ Links ]
7. Yang Z, Zhai W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn. Innov Food Sci Emerg Technol. 2010; 11(1): 169-176. [ Links ]
8. Mao S, Shuai X, Unger F. Simon M. Bi D. Kissel T. The depolymerization of chitosan: effects on physicochemical and biological properties. Int J Pharm. 2004; 281: 45-54. [ Links ]
9. Carlos-Salazar M, Valderrama-Negrón A. Preparación y caracterización de películas de quitosano despolimerizado y reticulado con tripolifosfato de sódio. Rev Soc Quím Perú. 2013; 79(3): 195-208. [ Links ]
10. Shu XZ, Zhu KJ. The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur J Pharm Biopharm. 2002; 54(2): 235-243. [ Links ]
11. Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963; 52(12): 1145-1149. [ Links ]
12. Korsmeyer RW, Gurny R, Doelker EM, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983; 15(1): 25-35. [ Links ]
13. Jing P, Ruan S, Dong Y, Zhang X, Yue J, Kan J. et al. Optimization of purification conditions of radish (Raphanus sativus L.) anthocyanin-rich extracts using chitosan. LWT – Food Sci Technol. 2011; 44(10): 2097-2103. [ Links ]
14. Gao R, Jing P, Ruan S, Zhang Y, Zhao S, Cai Z. et al. Removal of off-flavours from radish (Raphanus sativus L.) anthocyanin-rich pigments using chitosan and its mechanism(s). Food Chem. 2014; 146 (1): 423-428. [ Links ]
15. Pati F, Adhikari B, Dhara S. Development of chitosan–tripolyphosphate fibers through pH dependent ionotropic gelation. Carbohydr Res. 2011; 346(16): 2582-2588. [ Links ]
16. Salome CA, Godswill O, Ikechukwu OI. Kinetics and Mechanisms of Drug Release from Swellable and Non Swellable Matrices: A Review. RJPBCS. 2013; 4(2): 97-103. [ Links ]
17. Yoshida CM, Bastos CE, Franco TT. Modeling of potassium sorbate diffusion through chitosan films. LWT - Food Sci Technol. 2010; 43(4): 584-589. [ Links ]
18. Aldana AA, Gonzáles A. Strumia MC. Martinelli M. Preparation and characterization of chitosan/genipin/poly(N-vinyl-2-pyrrolidone) films for controlled release drugs. Mater Chem Phys. 2012; 134(1): 317-324. [ Links ]
Recibido el 03-05-17
Aprobado el 04-05-17