INTRODUCTION
Biomass has become in recent years an alternative source of molecular scaffolds for the synthesis of the next generation of bioactive compounds (nutraceuticals, antioxidants, antimicrobials, insecticides, drugs or materials). The presence of one or more chiral atoms in these metabolites increases their utility in the development of new drugs. Moreover, biomass processing often generates by-products useful in other industries1. A well-known example is the generation of 5-hydroxymethylfurfural (HMF) from the dehydration of fructose. The HMF can be transformed into different furan derivatives, such as 2,5-bis (hydroxymethyl) furan, which is an optimal substrate for the manufacturing of polyurethane foam with better flame hazards properties2.
Lichens are organisms widespread in nature, and are of particular interest in medicinal chemistry due to the wide variety of metabolites associated to their biomass. The use of xanthones has been reported for the synthesis of new molecules with better antifungal properties. Likewise, usnic acid, a major metabolite identified in many lichens, has been used to generate several derivatives with antimicrobial and tuberculostatic properties3,4.
Depsides are polyphenolic compounds comprising two or more monocyclic aromatic units, linked by an ester bond. They are often found in lichens, but have also been isolated from plants from the Ericaceae, Lamiaceae, and Papaveraceae family. Depsides have antibiotic, anti-HIV, and antiproliferative activities. As inhibitors of prostaglandin biosynthesis and leukotriene B4 biosynthesis, depsides are considered potent nonsteroidal anti-inflammatory compounds5.
The Parmeliaceae is a large and diverse family of Lecanoromycetes. A botanical study of all genera of Parmeliaceae family includes information on basic morphological characteristics for each species, but up to date there is no specific information regarding their phytochemical and pharmacological characteristics6. Previous research performed on the lichen Everniopsis trulla (Ach.) Nyl, (family Parmeliaceae), identified atranorin and usnic acid contained in the thallus surface. Tri-terpenes and β-orcinol depsidones were also found in its medulla6. In another study, the compounds chloratranorin and atranorin were identified from two extracts of E. trulla by TLC. The presence of two additional unknown substances was also detected7.
In this paper, the chemical structure of a new depside isolated from lichen E. trulla (Ach.)
Nyl is presented, which increases the knowledge about the metabolites present in this species.
EXPERIMENTAL PART
Sample.
The lichen specimen Everniopsis trulla (400 g) was collected in “Canchas city”, Asunción (Huaraz, Peru), in 2011 at an altitude of 3427 meters (10,650 feet). A voucher specimen was deposited in the Museum of Natural History of National University of San Marcos, UNMSM, Lima, Peru.
Extraction and isolation
The powdered sample generated from E. trulla, was extracted successively with ethanol The chloroform extract was run through a chromatographic column (silica gel, CHCl3- MeOH gradient) isolating five compounds: solid A (usnic acid), solid B (atranorin), solid C (trullarin), 2,4-dihydroxy-3-formyl-6-methylbenzoate (ethyl haematommate) and a compound still unidentified). Trullarin was purified by crystallization. Colourless single crystals were obtained by crystallization from EtOH/H2O (mp: 198-199 °C).
RESULTS AND DISCUSSION
Usnic acid, atranorin and 2,4-dihydroxy-3-formyl-6-methylbenzoate were identified by comparison of their spectroscopic data with the literature9,13,14.
Trullarin chemical structure elucidation
Trullarin was identified as depside through qualitative chemical reactions. This compound reacted with NaOCl and Ca(OCl)2 in solution to give red and orange colors; It also reacted with p-phenylenediamine to give red and orange colors. This reactivity is typical of a depside with an aromatic aldehyde moiety8,9. The chemical structure of trullarin was elucidated using 1H-NMR, 13C-NMR, UV and IR spectroscopies. The confirmation of the structure of trullarin was accomplished by single-crystal X-ray diffraction analysis.
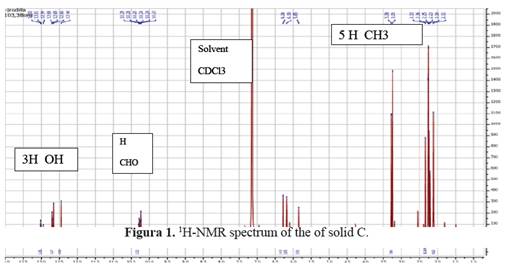
1H-NMR (benzene-d6, 400MHz): δ (ppm) 12.70, 12.65, 12.44 (each 1H, s, Ar-OH), 10.24 (1H, s, -CHO), 6.28 (1H, s, Ar-H), 3.25 (3H, s, - CO2Me), 2.33, 2.11 (each 3H, s, Ar-Me 5 and 6), 2.23 and 2.25 (each 3H, s, Ar-Me 3’ and 5’). See figure 1.
13C-NMR (benzene-d6, 100MHz): showed five methyl groups at δ9.4, 23.7, 24.0, 25.1 in two aromatic rings and 51.4 for the methyl ester. It also showed two carbonyl esters at δ 163.5 and 172.2 and showed an aldehyde group at δ 193.4 ppm in C-3. See figure 2.
MS: HRMS m/z 389.1624 (calcd for C20H21O8, 389.1622).
UV: λmax in ethanol 219.3 nm and 269.5 nm.
IR (KBr, cm-1): 3090, 3002, 1651, 1622, 1582, 1451, 1407, 1268, 1163, 1106, 820, 802, 610.
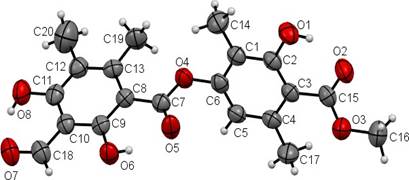
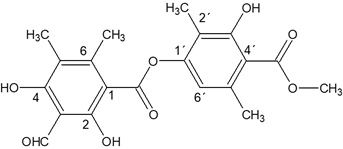
This compound called trullarin is new according to the PUCP BASE OF SCIENCE FINDER. Its chemical name is: (Methyl-2,6-dimethyl-4-(3-formyl-2,4-dihydroxy-5,6-imethylbenzoyloxy)-3'-hydroxybenzoate). HRESIMS (negative mode): m / z 387.1071 [M- H] + (calculated for C20H19O8: 387.1080). The title compound named trullarin, C20H20O8, crystallizes with one molecule in the asymmetric unit in the monoclinic centrosymmetric space group P21/n with the following unit cell parameters: a = 11.0400(3) Å, b = 11.2752(3) Å, c = 14.7893(4) Å, α = γ = 90°, β = 109.074(2)°, V = 1739.87(8) Å3, Z = 4. Figure 3 shows
the molecular structure in solid state, in which the individual conformation of trullarin can be observed. The crystallographic data is presented in Table 1. Selected bond lengths and angles are given in Table 2. All the bond lengths and angles are within the normal ranges.
Tabla 1 Crystallographic data for C20H20O8.
| CCDC | 1508526 |
| Empirical formula | C20H20O8 |
| Formula weight | 388.36 |
| Temperature (K) | 298(2) |
| Wavelength (Å) | 1.54178 |
| Crystal system | Monoclinic |
| Space group | P21 /n |
| Unit cell dimensions | |
| a (Å) | 11.0400(3) |
| b (Å) | 11.2752(3) |
| c (Å) | 14.7893(4) |
| α [º], β[º], γ[º] | 90, 109.074(2), 90 |
| Volume (Å3), Z | 1739.87(8), 4 |
| Density (calculated (Mgm-3) | 1.483 |
| Absorption coefficient (mm-1) | 0.975 |
| F(000) | 816 |
| Crystal size (mm) | 0.15 x 0.10 x 0.08 |
| Range for data collection θ (°) | 4.38 to 66.90 |
| Limiting indices h, k, l | -13≤ h ≤ 13, -12 ≤ k ≤ 13, -17 ≤ l ≤14 |
| Reflections collected | 11153 |
| Independent reflections | 2934 (R(int) = 0.0312) |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 2934 / 0 / 262 |
| Goodness-of-fit on F2 | 1.065 |
| Final R indices [I>2σ(I)] | R1 = 0.0678, wR2 = 0.2309 |
| R indices (all data) | R1 = 0.0819, wR2 = 0.2556 |
| Largest diff. peak and hole | 0.270 y -0.765 |
Tabla 2 Selected bond lengths (Å) and bond angles (º) for C20H20O8.
| Bond | Length (A) | Diedral angles | Angles (°) |
|---|---|---|---|
| O(1)-C(2) | 1.353(3) | C(15)-O(3)-C(16) | 117.3(3) |
| O(2)-C(15) | 1.227(4) | C(7)-O(4)-C(6) | 117.8(2) |
| O(3)-C(15) | 1.308(4) | O(1)-C(2)-C(3) | 122.6(3) |
| O(3)-C(16) | 1.449(4) | O(1)-C(2)-C(1) | 115.0(2) |
| O(4)-C(7) | 1.345(3) | C(1)-C(6)-O(4) | 117.4(3) |
| O(4)-C(6) | 1.414(3) | C(5)-C(6)-O(4) | 118.5(3) |
| O(5)-C(7) | 1.212(3) | O(5)-C(7)-O(4) | 120.7(3) |
| O(6)-C(9) | 1.328(3) | O(5)-C(7)-C(8) | 124.3(2) |
| O(7)-C(18) | 1.227(4) | O(4)-C(7)-C(8) | 115.0(2) |
| O(8)-C(11) | 1.336(3) | O(6)-C(9)-C(10) | 115.3(2) |
| C(7)-C(8) | 1.468(4) | O(6)-C(9)-C(8) | 123.5(3) |
| O(8)-C(11)-C(12) | 118.9(3) | ||
| O(8)-C(11)-C(10) | 120.8(3) | ||
| O(2)-C(15)-O(3) | 121.7(3) | ||
| O(2)-C(15)-C(3) | 122.7(3) | ||
| O(3)-C(15)-C(3) | 115.6(2) | ||
| O(7)-C(18)-C(10) | 123.4(3) | ||
The planar conformation of the ester groups is established from the torsion angles O3-C15- O2-C3 = -178.5(5) and O4-C7-O5-C8 = 179.3(5). The hydroxyl groups are coplanar with the aromatic rings; the O1-C2-C1-C6, O6-C9-C10-C11 and O8-C11-C12-C13 torsion angles are -179.9 (3)°, 179.5 (3)° and -179.6 (3)°, respectively.
In the crystal structure of trullarin, there are two benzene rings in the molecule. C(1), C(2), C(3), C(4), C(5) and C(6) form the first plane, with a mean deviation of 0.0167 Å, defined as plane I. Similarly, C(8), C(9), C(10), C(11), C(12) and C(13) forms the second plane with a mean deviation of 0.0048 Å, defined as plane II. The dihedral angle between plane I and plane II is 64.4(9)º. In addition, the ester group C(7), C(8), O(4) and O(5) forms a third plane, defined as plane III. The dihedral angle between plane II and plane III is 4.3(1)º, which suggests that both are nearly co-planar. This indicates that there is a π-π conjugation between the C(=O) group and the benzene ring of plane II15,16.
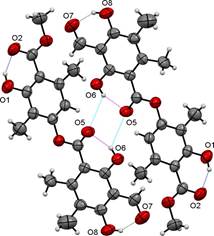
The molecular packing of trullarin is influenced by both intramolecular and intermolecular forces. Intramolecular forces determine molecular shape, which in turn play an important role in determining the most effective ways of packing the molecules in the crystal [13]. In the structure, there are three intramolecular hydrogen bonds of O-H···O type. The O1···O2, O5···O6 and O7···O8 intramolecular hydrogen bonds generate a six-membered rings (Table 3 and Fig. 4). Furthermore, one intermolecular hydrogen bond of O-H···O type, O5·O6, links adjacent molecules into dimers (Table 3 and Fig. 4). The dimers form twelve-membered rings and present an anti-parallel orientation along an axis. The packing diagram in Fig. 5 shows the stacking pattern of trullarin, viewed along the [010] direction. The chemical structure obtained by single crystal XRD agrees very well with NMR spectroscopy data.
Tabla 3 Intra- and Intermolecular hydrogen bonds geometry (Å, º) for C20H20O8.
| D-H···A | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|
| O1-H1···O2i | 0.82 | 1.81 | 2.540(3) | 147 |
| O6-H6···O5i | 0.82 | 1.83 | 2.541(3) | 144 |
| O8-H8···O7i | 0.82 | 1.83 | 2.554(4) | 146 |
| O6-H6···O5ii | 0.82 | 2.52 | 3.088(3) | 127 |
Symmetry codes: (i) = x, y,z (ii) = 1-x, 1-y, -z
The chemical structure obtained by single crystal XRD aligns very well with NMR spectroscopy data. Figure 6, shows the chemical structure of the isolated new compound from E. trulla.
CONCLUSIONS
Four compounds were isolated from the Peruvian Lichen E. trulla. One of them, compound C (trullarin), is reported for the first time. Has melting point of 198 ° C were obtained, whose structure was elucidated based on spectroscopic data (UV-Visible, IR, NMR-H1, NMR-C13, mass spectrometry and single crystal X-ray diffraction) and its chemical name is: (Methyl- 2,6-dimethyl-4 '- (3-formyl-2,4-dihydroxy-5,6-imethylbenzoyloxy) -3'-hydroxy benzoate).