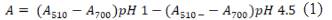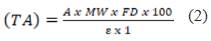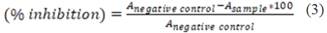INTRODUCTION
Free radicals are a molecular species with an unpaired electron in an atomic orbital, causing them to be highly reactive and capable of altering different molecules in the body, resulting in the progression of a number of diseases and also in aging1. There are many sources of free radicals, including enzymes such as xanthine oxidase, cyclooxygenases, lipoxygenases, myeloperoxidases, cytochrome P450 monooxygenase, uncoupled nitric oxide synthase (NOS), peroxidases and NADPH oxidase2.
Antioxidants can prevent cellular damage resulting from chemical reactions of free radicals by neutralizing and stabilizing them3. Different studies have reported the effects of natural antioxidants in diseases related to stress oxidation, such as cancer or atherosclerosis4,5.
Blackberries belong to the genus Rubus of the rose family (Rosaceae), it is a berry fruit which consists of numerous black juicy drupelets, each with a single seed around a central stem. It is commonly found in North America and Europe. Blackberries are eaten fresh or in other food products such as ice cream, jams and pies6. Blackberries are known to be rich in anthocyanins and other phytochemicals, such as flavonols, phenolic acids, ellagic acid, vitamins C and E, folic acid and b-sitosterol7. The phenolic composition, antioxidant capacity and anthocyanin content of different species of blackberries8,9,10,11,12,13 have been studied using different determination techniques most of all concluding that this fruit has an antioxidant capacity due to their high anthocyanin content, more precisely from cyanidin-3-glucoside14. In Peru, blackberries are poorly grown and often imported but this fruit can grow by itself like tare with a source of water nearby.
We emphasize the importance of publishing information about blackberries as antioxidants in Peru since there are few reports available on the antioxidant capacity of this fruit in this region. This research will be helpful for greater understanding the value of blackberries to the health of Peruvian consumers and for encouraging its consumption. The aim of this research study, therefore, was to determine total anthocyanins (TA), total phenols (TP) and antioxidant capacity of Rubus robustus C. Presl (wild blackberries) in Arequipa, Peru.
EXPERIMENTAL
This research was carried out in the Proyecto Mercurio Laboratory (H-202) at the Universidad Católica de Santa María and in the Calidad de Aguas Laboratory at the Universidad Tecnológica del Perú in Arequipa.
Fruit Sample
Mature wild blackberries were collected from adult wild plants in the Chilina Valley in the spring (between September and October) as shown in Figure 1. Gathering was carried out by direct hand harvesting of the fully ripe fruits and they were selected by uniformity of size and color and stored in polyethylene terephthalate (PET) containers. Fruit samples were sent to the Proyecto Mercurio Laboratory, where they were carefully washed with deionized water and kept refrigerated at 4 ± 1 °C. Later, the samples were blended and filtrated to eliminate any solid material before being freeze-stored. Afterwards, the samples were lyophilized in a FreeZone 2.5 Liter Benchtop Freeze Dry System at the Universidad Nacional San Agustin of Arequipa. Finally, the lyophilized samples were placed in PET containers and stored in an environment free of humidity and protected from direct light. A sample of the adult plant and fruit were sent to the Herbarium Areqvipense at the Universidad Nacional San Agustin of Arequipa, which identified it as Rubus robustus C. Presl.
Sample preparation
The lyophilized sample (100 mg) was treated with 1 mL of high-performance liquid chromatography (HPLC) grade methanol and sonicated in a Branson 2510-E sonicator for 10 min. The homogenate was then centrifuged in a Centra CL 2 Thermo IEC centrifuge at 4000 rpm for 15 min at room temperature. The supernatant was separated and the remaining insoluble fraction was washed again with 1 mL of HPLC grade methanol. This process was repeated three times and the supernatants were combined. Finally, a final volume of 10 mL was obtained with HPLC grade methanol and maintained at 4 ± 1 °C.
Reagents
Follin-Ciocateau reagent P.A (Merck), gallic acid P.A (Sigma Aldrich), sodium carbonate P.A (Merck), potassium chloride P.A (Merck), sodium acetate P.A (Merck), 6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid (Trolox) standard (CALBIOCHEM), DPPH P.A
(CALBIOCHEM), HPLC grade methanol (MERCK), deionized water (18.2 mΩ) obtained from a Barnstead Easypure II water purification system.
Determination of total anthocyanins
The pH differential method has been used by many authors to determine the anthocyanins of berries11,12,13 where a change in pH, anthocyanins go through a reversible structural transformation demonstrated by different absorbance spectra. At pH 1.0, the red oxonium form appears and at pH 4.5 the colorless hemiketal form predominates15. Absorbance was measured with a CARY60 UV -VIS spectrophotometer (Agilent Technologies) at 510 and 700 nm at different pHs to calculate the final absorbance using Eq. 1
The TA was calculated according to Eq. 2:
Where: A= Absorbance, MW= molecular weight, DF= dilution factor, ε= extinction coefficient. Data were calculated with the MW (449.20) and the extinction coefficient for cyanidin-3-glucoside (29 600) and expressed as mg cyanidin/100 g lyophilized Rubus robustus C. Presl.
Determination of total phenolics compounds
The TP was determined by the Folin-Ciocalteu colorimetric method, first described by Singleton and Rossi (1965)16 based on the production of a blue phosphotungstic- phosphomolydic complex. This method is widely used to estimate the total phenolic content in many samples such as fruits, beverages, herbs and other plant extracts17,16,19.
A calibration curve was prepared with gallic acid ranging from 10 to 60 ppm. Different concentrations of gallic acid were mixed with 0.25 mL of Folin-Ciocalteu reagent in a 10 mL volumetric flask at room temperature. Then, 2 mL of a 20% sodium carbonate solution was added and finally deionized water was added to complete 10 mL. This solution was mixed in an Analog mixer vortex and maintained for 2 h in dark conditions. Finally, total sample phenols were evaluated with a CARY60 UV -VIS spectrophotometer (Agilent Technologies) at 750 nm.
For the determination of TP of the fruit sample, the same procedure was done using 0.2 mL of the sample previously prepared. TP was expressed as mg of gallic acid equivalent (GAE)/100 g lyophilized Rubus robustus C. Presl.
Antioxidant assay: Determination of the DPPH free radical scavenging activity
The DPPH free radical method is based on the measurement of the scavenging capacity of antioxidants towards a free radical20 and it is used in various studies to determine the antioxidant capacity of plants21,22,23. When a DPPH solution is mixed with a substance such as an antioxidant that can donate a hydrogen atom, it gives rise to a reduced form with the loss of its violet color24.
A stock solution of Trolox at a concentration of 1 mmol/L was prepared with HPLC grade methanol in a 10 mL volumetric flask and stored in the fridge until use. A working solution of DPPH at a concentration of 2.5 mmol/L was prepared with HPLC grade methanol and was kept in dark conditions to prevent its deterioration. This solution was prepared on each day of the analysis.
A calibration curve was prepared using different volumes of the stock solution of Trolox to obtain a concentration range of 0.02-0.10 mmol/L, 100 uL of the DPPH working solution and HPLC grade methanol to achieve a volume of 1 mL. To prepare the negative control, DPPH was mixed with methanol instead of Trolox. Finally, for the analysis of the sample, two different volumes (100 and 200 uL) of the fruit samples prepared earlier were taken, 100 uL of the DPPH and HPLC grade methanol were added to obtain a volume of 1 mL and filtrated in an Anotop filter before injecting them to the HPLC. The absorbance was measured at 517 nm every 20 min. The samples and standards were analyzed. The peaks identification was performed by the comparison of the retention times with those of referential standards.
The analysis was performed on a LaChrom-Hitachi HPLC, consisting of an L-7100 pump, a 20 uL manual injector (loop) and a L-7400 UV detector. An EZ Chrom Elite data station and a personal computer were used as software sources for data storage and evaluation. The analytical column used was a Chromolith RP-18e of 100-4.6 mm, with internal particle diameter of 2 um. A 5-4.6 mm Chromolith RP-18e pre-column was used to protect the analytical column. Chromatographic separation was performed using, as a mobile phase methanol: water (80:20) with a total flow rate at 1.0 mL/ min. An absorbance detector was used at 517 nm.
The results were expressed as a percentage decrease of color intensity (% inhibition of the free radical) related to the negative control sample such as Eq. 3:
where: Anegative = area of the negative control and Asample = area of the sample.
Determination of the IC50
The IC50 is the concentration of an antioxidant that inhibits by 50% the DPPH free radical activity. The IC50 is inversely proportional to the antioxidant activity where a lower value indicates a higher effectiveness of the antioxidant23. To determine the IC50, a calibration curve was prepared using serial dilutions made from the stock solution of the fruit sample to obtain solutions with concentrations of 3000, 2000, 1500, 1000, 800, 500 and 200 µg/mL, mixing them with 100 uL of the DPPH working solution and HPLC grade methanol. The working solution of DPPH 2.5 mmol/L was prepared as previously explained. To prepare the negative control, DPPH was just mixed with methanol. Finally, the absorbance was measured at 517 nm on a GENESYS UV -VIS spectrophotometer (Thermoscientific)
Statistical analysis
The data obtained was analyzed on the basis of a random design using the general linear model with five replicates and by analysis of variance (ANOVA). All statistical tests were performed with a level of significance of 0.05. Significant differences among means were determined by Tukey´s test at 5% level of significance.
RESULTS AND DISCUSION
Determination of TA and TPC
Total anthocyanins and total phenolic contents are presented in Table 1. Lyophilized Rubus robustus C. Presl presented 52 ± 1.86 mg of total anthocyanins whereas Croge et al. (2019)12, presented a value between 80 a 134 mg /100 g of fresh fruit for different blackberries species hence the fruit extract possesses a higher anthocyanin content possibly due to the extraction method. What is more, in a nearby country such as Brazil, the content of phenolic compounds in blackberry was reported with values ranging 234 to 800 mg GAE/ 100 mg of fresh fruit12,13,14,15,16,17,18,19,20,21,22,23,24,25, where as the lyophilized sample of Rubus robustus C. Presl presented 219.11 ± 8.23 mg GAE/100 mg indicating the climate and the temperature influences in the content of these compounds. In addition, in Peru, values of TPC for Rubus fructicosus L 400.67 mg GAE /100 g of fresh fruit were found26 showing a difference with the lyophilized Rubus robustus C. Presl which could be due to the species of Rubus, the type of sample and the growing conditions.
Antioxidant assay: Determination of the DPPH free radical scavenging activity
Eq. 3 was used to calculate the percentage of inhibition of DPPH. Table 2 presents the results of the percentage of inhibition of DPPH in 5 different repetitions using different concentrations of Trolox. As seen in Table 2, if the concentration of the antioxidant increases, so does the percentage of inhibition of the free radical.
Tabla 2 Percentage of inhibition of DPPH using different concentrations of Trolox.
| Concentrations of Trolox (mmol/mL) | Percentage of inhibition (%) |
| 0.02 | 13.67 ± 3.34 |
| 0.04 | 28.35 ± 5.43 |
| 0.06 | 42.81 ± 7.06 |
| 0.08 | 56.4 ± 6.04 |
| 0.1 | 71.96 ± 7.67 |
Results show that 1.0 and 2.0 mg/mL of the lyophilized sample present a percentage of inhibition of 35.89 ± 4.43 and 67.23 ± 3.30%, respectively. In another study, the DPPH discoloration percentages of different fresh berries was determined, where wild blackberries had a percentage of 87.5%27, showing that fresh blackberries have more antioxidant capacity than the lyophilized samples in this study with 67%. Furthermore, in a study conducted with Rubus laciniatus cv. Hull (thornless blackberry) in China, at 2 mg/mL, blackberry extracts could scavenge nearly all DPPH radicals (95.37%)28 whereas in Peru, with Rubus robustus C. Presl at the same concentration, the percentage of inhibition was 67%, demonstrating a difference in the species and in the location. What is more, the scavenging capacity of five blackberry cultivars produced in Brazil29 was determined, ranging from 53 to 74%, proving that Rubus robustus C. Presl has an average antioxidant capacity.
Figure 2 illustrates the chromatograms of three samples: A) DPPH + methanol, B) DPPH  + methanol + Trolox (0.06 mmol/mL) and C) DPPH + methanol + sample (1.0 mg/mL). As seen in these chromatograms, the retention time is approximately 2.91 min and the total runtime was 5 min. When comparing chromatogram A and B, chromatogram B shows that a Trolox concentration (in this case 0.06 mmol/mL) decreases the area of the DPPH peak, mainly because the Trolox solution is used as an antioxidant. In chromatogram C, the sample is proven to contain antioxidant capacity as the peak area of DPPH decreases compared to chromatogram A, with an effect similar to that of the Trolox solution used.
+ methanol + Trolox (0.06 mmol/mL) and C) DPPH + methanol + sample (1.0 mg/mL). As seen in these chromatograms, the retention time is approximately 2.91 min and the total runtime was 5 min. When comparing chromatogram A and B, chromatogram B shows that a Trolox concentration (in this case 0.06 mmol/mL) decreases the area of the DPPH peak, mainly because the Trolox solution is used as an antioxidant. In chromatogram C, the sample is proven to contain antioxidant capacity as the peak area of DPPH decreases compared to chromatogram A, with an effect similar to that of the Trolox solution used.
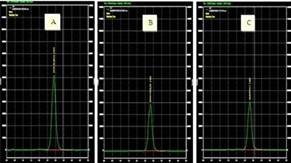
Figura 2 Chromatograms: A) DPPH + methanol, B) DPPH + methanol + Trolox (0.06 mmol/ mL) and C) DPPH + methanol + sample (1.0 mg/mL blackberry sample)
Determination of the IC50
Table 3 summarizes the DPPH radical scavenging activity of lyophilized Rubus robustus C. Presl. All experiments were conducted in triplicate (n=3) and reported as the mean of three values together with standard deviation (±SD). The IC50 value for lyophilized Rubus robustus C. Presl was found to be approximately 1.5 mg/mL hence proving that this species of blackberry in Peru possess an antioxidant activity.
| Concentrations (µg/mL) | Percentage of inhibition (%) |
|---|---|
| 200 | 10.39 ± 3.15 |
| 500 | 19.18 ± 1.10 |
| 800 | 25.24 ± 0.95 |
| 1000 | 38.15 ± 4.64 |
| 1500 | 53.52 ± 2.60 |
| 2000 | 57.06 ± 3.56 |
| 3000 | 58.42 ± 2.10 |
Other blackberries species like the Brasilian Blackberry Xavante and Blackberry Cherokee have demonstrated a high IC5025. In addition, when comparing different small berries in Italy, blackberries have evidenced to be one of the best natural antioxidants13.
CONCLUSIONS
Wild blackberries collected from the Chilina Valley in Arequipa, Peru, exhibited antioxidant capacity demonstrated by their content of anthocyanins (52 ± 1.86 mg cyanidin 3-glucoside/100 g of lyophilized Rubus robustus C. Presl) and phenols (219.11 ± 8.23 mg gallic acid/100 g of the lyophilized fruit) as well as by the antioxidant assay with a percentage of 35.89 ± 4.43 and 67.23 ± 3.30%, respectively. In addition, the IC50 value found was 1.5 mg/mL. A diet rich in this fruit could provide a good source of antioxidants, and therefore it should be encouraged in Peru. Blackberries are not currently extensively grown in Arequipa, but their consumption may have potential for use in the development of nutraceuticals for regional and international markets. Furthermore, they could be used as functional food ingredients or supplements as a benefit to human health. Further studies should be done to compare the antioxidant capacity in cultivated and wild blackberries in the same area and in other areas of Arequipa, Peru.