Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Scientia Agropecuaria
Print version ISSN 2077-9917
Scientia Agropecuaria vol.6 no.4 Trujillo Oct. 2015
http://dx.doi.org/10.17268/sci.agropecu.2015.04.08
ARTÍCULOS ORIGINALES
Maximizing content of Omega-3 (EPA and DHA) in the process of enzymatic acidolysis of canola oil and concentrated of long-chain polyunsaturated fatty acids (LCPUFA) in supercritical CO2 conditions
Maximización del contenido de Omega-3 (EPA y DHA) en el proceso de acidólisis enzimática de aceite de canola y concentrado de ácidos grasos poliinsaturados de cadena larga (AGPICL), en condiciones de CO2 supercrítico
José Cedano Romero1; Alicia Rodríguez1; Raúl Siche2
1 Departamento de Ciencia de los Alimentos y Tecnología de los Alimentos, Facultad de Ciencias Químicas y Farmacéuticas, Universidad de Chile, Chile.
2 Instituto Regional de Investigación Agraria (IRIA), Universidad Nacional de Trujillo, Av. Juan Pablo II s/n. Ciudad Universitaria, Trujillo, Peru.
Abstract
The aim of this study was to optimize the content of EPA and DHA in the process of enzymatic acidolysis of canola oil and concentrated of long-chain polyunsaturated fatty acids (LCPUFA) in structured triacylglycerols (TAGs). For this purpose, nonspecific lipase B from Candida antarctica immobilized in a supercritical CO2 was used. Crude salmon oil obtained from the industrial byproducts was used to obtain LCPUFA concentrate. Initially, a LCPUFAs concentrate was obtained by basic hydrolysis and posterior complexation with urea. Subsequently the process variables were optimized enzymatic acidolysis were optimized using a central composite rotational design 25-1 + star, with 5 factors and 30 experimental trials, based on the response surface methodology. The optimal conditions that maximized the content of EPA and DHA to 3.92 g/100 g TFA and 9.09 g/100 g TFA, respectively in the purified TAGs corresponded to a LCPUFA percentage 71.71% and canola oil percentage 28.29%, temperature 57.8 °C, pressure 172.0 bar, time 23.97 h enzyme percentage of 7.74%.
Keywords: Optimization, supercritical carbon dioxide, structured triacylglycerols, docosahexaenoic acid (DHA), eicosapentaenoico acid (EPA).
Resumen
El objetivo del presente trabajo fue optimizar el contenido de ácidos grasos EPA y DHA en el proceso de acidólisis enzimática de aceite de canola y concentrado de ácidos grasos poliinsaturados de cadena larga (AGPICL) en triacilglicéridos estructurados (TAGs). Para ello, se empleó lipasa B inespecífica de Candida antarctica inmovilizada en condiciones CO2 supercrítico. El aceite crudo de salmón obtenido a partir de los subproductos industriales se utilizó para obtener concentrados de AGPICL. Como primer paso, se obtuvo un concentrado de AGPICL mediante una hidrolisis básica y posterior complejación con urea. Posteriormente se optimizó las variables del proceso de acidólisis enzimática mediante un diseño compuesto central rotacional 25-1 más estrella, de 5 factores con 30 ensayos experimentales, basado en la metodología superficie respuesta. Las condiciones óptimas que maximizaron el contenido de EPA a 3,92 g/100 g de ácidos grasos totales (AGT) y de DHA a 9,09 g/100 g AGT en los TAGs purificados correspondieron a una relación AGPICL/Canola de 71,71 %, temperatura de 57,8 ºC, presión de 172,0 bar, tiempo de 23,97 h y concentración de enzima de 7,74%.
Palabras clave: Optimización, dióxido de carbono supercrítico, triacilglicéridos estructurados, ácido eicosapentaenoico (EPA), ácido docosahexaenoico (DHA).
1. Introduction
Western diets are deficient in long-chain polyunsaturated fatty acids (LCPUFA) (Omega 3 (n-3)) of marine origin (such as eicosapentaenoic acid – EPA – and docosahexaenoic acid – DHA); consumption is less than intake recommendations and food requirements to prevent clinical deficiencies, provide optimal health and reduce the risk of developing chronic diseases. Furthermore, excessive amounts of polyunsaturated fatty acids omega-6 (n-6) and a high proportion of n-6/n-3, as found in Western diets today promote pathogenesis of many diseases, including cardiovascular diseases, cancer, inflammatory and autoimmune diseases, while higher than the highest levels of n-3 LC-PUFA and a low proportion of n-6/n-3 exert protective effects (Simopoulos, 2002).
The LCPUFA n-3, such as EPA and DHA, have been identified as responsible for the beneficial effects on human health related to the cardiovascular system, central nervous system development of the brain and retina (Neuringer et al., 1986; Burr et al., 1989; Simopoulos, 1991; Uauy-Dagach y Valenzuela, 1992; Uauy y Valenzuela, 2000; Ramírez, 2006; Araya, 2008; FAO, 2012).
The structured triacylglycerol (TAGs) are those that have modified their composition and/or positional distribution of fatty acids in the glycerol molecule through chemical and/or enzymatic methods, with improved functional properties and/or nutritional (Osborn y Akoh, 2002). In this regard, the enzymatic transesterification provides multiple advantages, as the use of relatively low temperatures and increased catalyst selectivity. The supercritical carbon dioxide (CO2) is the most suitable solvent for enzymatic catalysis; since it exhibits similar to those of organic solvent properties, but with additional features to promote transport phenomena and facilitate separation post-reaction due to varying solvent power, which makes it more attractive. In addition, its critical pressure of 7.4 MPa and critical temperature of 31 °C is low enough for the food processing thermolabile as easily oxidized fats. Also, it is a clean, non-toxic and friendly technology environment.
A food and nutrition level, strategies should focus on increasing consumption of n-3 PUFAs in the population, especially when you consider that the Western diet is poor in them, which should encourage consumption of foods rich EPA and DHA, mainly fatty fish, or develop functional foods that contain and / or nutritional supplements (nutraceuticals) with n-3 PUFA (Valenzuela et al., 2011).
By the above, the aim of this investigation was to optimize the content of EPA and DHA fatty acids in the structured triglycerides, obtained from enzymatic acidolysis process canola oil and concentrated LCPUFA able dioxide supercritical carbon.
2. Materials and methods
2.1. Raw material
Crude salmon oil was provided by the company Salmonoil S.A. (Puerto Montt, Chile). The Mazola oil 100 % canola (Superior Quality, E. 12.06.14/16) was purchased from Watt’s (Santiago, Chile). The internal standard used was metil tricosanoato (23:0) (Nu-Check-Prep, Elysian, MN, USA). Reference Standard GLC-463 (Nu-Check-Prep, Elysian, MN, USA). Urea, ethanol, n-hexane, methanol and α-tocopherol were obtained from Merck (Santiago, Chile).
2.2. Obtaining LCPUFA concentrated
Preparing of long-chain polyunsaturated fatty acids (LCPUFA) concentrated from crude salmon oil was performed by basic hydrolysis and subsequent inclusion in urea crystals. Thus, 500 g of crude salmon oil were weighed and mixed with a solution of KOH. The mixture at 60 °C under reflux for 90 minutes was subjected, with constant stirring under nitrogen. After saponification, two extractions were conducted with hexane; the combination of both was filtered through anhydrous Na2SO4 to remove moisture. Finally, the solvent was evaporated in vacuum and the remaining solvent was removed with a stream of nitrogen gas. Free fatty acids (FFA) obtained after basic hydrolysis were stored at -80 °C until use (Wanasundara y Shahidi, 1999). The free fatty acids were mixed with a solution of urea in 95% ethanol in an Erlenmeyer flask. The solution was placed in a rod system with reflux and heated to 60 °C. Then it is brought to a temperature of -23 °C for 22 hours with constant stirring. The crystals formed are separated from the liquid under gravity filtration with Whatman Qualitative Grade 1 Filter Paper and is received in a flask. The filtrate was diluted with 900 ml of distilled water and acidified to pH 4.5. Hexane extractions are performed, the phases were separated and the aqueous phase was removed. The solvent was removed at 40 °C using a rotary evaporator and with a stream of nitrogen gas remaining solvent was removed. LCPUFA concentrated was stored at -80 °C with 100 ppm of α-tocopherol until analysis (Pando et al., 2014).
2.3. Experimental design
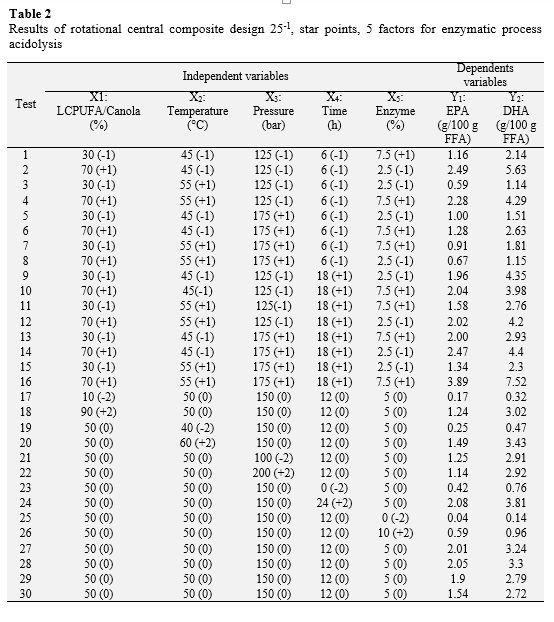
A rotatable central composite design 25-1 (star points), 5 factors, based on the methodology of response surface was used to study the effect of the variables and maximize the content of EPA and DHA by enzymatic acidolysis of canola oil and concentrated LCPUFA. The independent variables were: relationship LCPUFA / Canola (X1), reaction temperature (X2), reaction pressure (X3), reaction time (X4) and enzyme concentration (X5) with respect to the substrate. Also, the dependent variables were the contents of EPA (Y1) and DHA content (Y2). 30 trials with 5 levels (-2, -1, 0, 1, 2) which included 4 replications at the center point (0, 0, 0, 0, 0), in order to estimate experimental error were performed. All experiments were performed in random order.
2.4. Enzymatic hydrolysis in super-critical CO2
Thirty enzymatic hydrolysis experiments were performed on a Spe-edTM SFE Applied Separation equipment with fluid carbon dioxide (CO2) in supercritical conditions. In the reactor column 10 g sample (concentrated mixture LCPUFA, canola oil and enzyme varied by experimental design) were deposited. Subsequently, the reaction according to the temperature, pressure and time established by the experimental design was scheduled. The obtained samples were stored at -80 °C for later analysis.
2.5. Purification of structured triacylglycerols
The reaction products from the enzymatic hydrolysis (mainly structured triglycerides and free fatty acids) were purified by neutralization of free fatty acids with 1N NaOH in ethanolic solution (ratio ethanol/water 50/50 v/v) and then drawing structured triacylglycerols in the hexane phase (Jimenez et al., 2010).
2.6. Analysis of reaction products
Identifying the products of enzymatic hydrolysis reaction (structured triacylglycerides and free fatty acids) it was performed using TLC (thin - layer Chromatography, TLC). Each of these lipid components were identified by TLC on silica gel plates (TLC Silica gel 60 - Merck Millipore). The mobile phase used was chloroform / acetone / methanol (95:4.5:0.5 v/v/v) (Robles et al., 2011). Four µL of sample was seeded (previously, 1:25 sample was diluted in hexane) and allowed to elute the solvent together with the analytes. The plate was dried and left in a closed chamber with iodine solution so that the vapors made contact with the plate and dyed different lipid components (Robles et al., 1999).
2.7. Characterisation of fatty acid
The fatty acid profiles of canola (Brassica napus L.), crude oil commercial salmon LCPUFA and concentrated products 30 experiments were characterized.
The fatty acids of the samples became fatty acid methyl esters (FAME) by methylation using hot sodium methoxide followed by acidic (IUPAC Standard Method 1987 2.301). The internal standard used was methyl tricosanoato (23:0) (Nu-Check-Prep, Elysian, MN, USA). The FAME were analyzed using a gas chromatograph HP 5890 series II (Palo Alto, California, USA), with flame ionization detector (FID), injection system Split and capillary column of fused silica SPTM - 2560 of 100 m x 0.25 mm x 0.2 μm film thickness (Supelco, Bellefonte, PA, USA). The following parameters were used: Temperature of injector and detector 250 °C, initial oven temperature 100 °C, at 3 °C/min to 140 °C, then the temperature was increased to 170 °C at 0.5 °C/min, finally increased to 220 °C at 4 °C/min and held at 220 °C for 30 min (total time 115.83 min). Hydrogen carrier gas was used high purity (Linde, Chile) 1 µL injection volume. The FAME were identified by comparison with the reference standard GLC-463 (Nu-Chek-Prep, Elysian, MN, USA) and quantification was performed according to standard AOCS Official Method (AOCS 2009, Ce 1j-7). The software used for analysis of the chromatograms was DataApex ClarityTM (DataApex Ltd., Prague, Czech Republic).
2.8. Characterization of raw salmon oil and canola oil
The characterization for crude oil and salmon oil canola was performed using the following methods: free fatty acids – FFA (AOCS Official Method Ca 5a-40, 1993), Peroxide value (AOCS Official Method Cd 8b-90, 1993), p-anisidina value (AOCS Official Method Cd 18-19, 1993) and TOTOX value (Wanasundara y Shahidi, 1995).
3. Results and discussion
3.1. Obtaining LCPUFA concentrated
In the basic hydrolysis, the yield of FFA obtained was 94.2 ± 1.9% compared to crude salmon oil and the process including urea crystals, the yield of the concentrate obtained LCPUFA was 6.8 ± 0.9% with respect to FFA after basic hydrolysis.
3.2. Characterization of fatty acid profiles
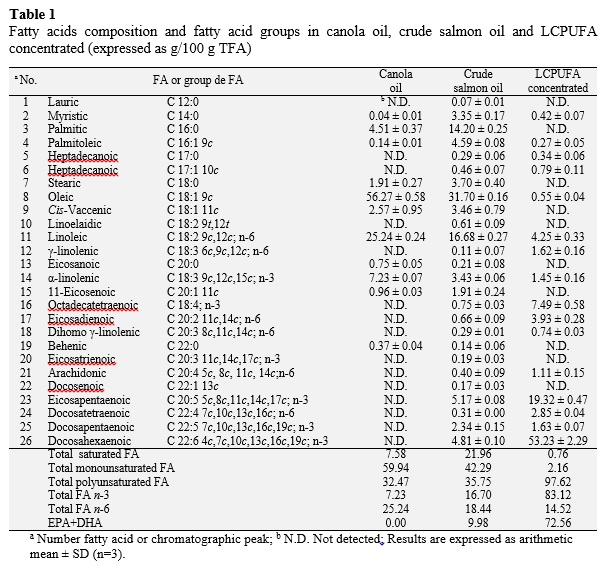
The main fatty acid (FA), expressed in g/100 g FFA and percentage of methyl esters of total fatty acids (TFA), present in the canola oil was oleic acid (56.27, 56.22%). Other FA present in the canola oil were linoleic acid (25.24, 25.12%), linolenic acid (7.23, 7.42%) and palmitic acid (4.51, 4.52%). The content of saturated FA presented a value of 7.58 (7.60%), in monounsaturated FA was 59.94 (59.87%) and polyunsaturated FA 32.47 (32.54%) (Table 1). Masson and Mella (1985) determined for canola oil, the content of saturated FA, expressed as a percentage of methyl esters, has a value of 7.4%, the content of monounsaturated and polyunsaturated FA present values of 65.8% and 26.7%, respectively. Ghazani and Marangoni (2013) disclose that canola oil contains between 6% and 14% of α-linolenic acid, between 50% and 66% oleic acid and less saturated FA amount (< 7%). The most abundant fatty acids found in salmon were crude oil (g/100 g TFA): C 14:0 (3.35), C 16:0 (14.20), C 16:1 9c (4.59), C 18:0 (3.70), C 18:1 9c (31.70), C 18:1 11c (3.46), C 18:2 n-6 (16.68), C 18:3 n-3 (3.43), C 20:5 n-3 (5.17), C 22:5 n-3 (2.34), C 22:6 n-3 (4.81). Regarding the composition in g/100 g TFA of saturated FA they had a value of 21.96, the monounsaturated FA a value of 42.29 and polyunsaturated FA containing 35.75. The composition of fatty acids in the LCPUFA concentrated was (g/100 g TFA): C 14:0 (0.42), C 16:1 9c (0.27), C 18:1 9c (0.55), C 18:2 n-6 (4.25), C 18:3 n-3 (1.45), C 20:5 n-3 (19.32), C 22:4 n-6 (2.85), C 22:5 n-3 (1.63), C 22:6 n-3 (53.23). Regarding the composition, values of 0.76, 2.16 and 97.62 g/100 g TFA are obtained for saturated, monounsaturated and polyun-saturated FA, respectively. The saturated fatty acids decrease after inclusion crystals with urea (from 21.96 to 0.76 g/100 g TFA), while the content of unsaturated fatty acids increased, especially in the case of n-3 PUFA. Saturated fatty acids of crude oil as C 12:0, C 14:0, C 16:0, C 18:0, C 20:0 and C 22:0, and monounsaturated fatty acids, such as C 16:1 9c, C 18:1 9c, C 18:1 11c, C 20:1 11c, C 22:1 13c, form adducts due to the inclusion of urea and were retained in the disposable phase.
The content of n-3 PUFA (g/100 g TFA) of crude oil and concentrate LCPUFA salmon it was of 16.70 and 83.12, respectively (Table 1). The PUFA-n-3 increases in the concentrate LCPUFA because they remained in the fraction not complexed with urea while the content in saturated fatty acids and monounsaturated fatty acids decreased because formed adducts with urea (Table 1). This method preferably used by researchers because complex formation depends on the configuration of fatty acids due to the presence of multiple double bonds, rather than pure, such as melting point or solubility physical properties (Wanasundara, 1996). Fractionation of fatty acids with urea is mainly based on the degree of unsaturation; Thus, the more unsaturated, less is included in crystals of urea (Haagsma et al., 1982). This is a well-established technique for the removal of saturated and monounsaturated fatty acids (Iverson and Weik, 1967; Strocchi and Bonaga, 1975). In the case of fatty acids C 20:5 n-3 (EPA) and C 22:6 n-3 (DHA) for crude salmon oil, content was 5.17 and 4.81 g/100 g TFA, respectively, content those fatty acids (EPA and DHA) increases after urea complexation process with a value of 19.32 and 53.23 g/100 g TFA respectively in the concentrated LCPUFA (Table 1). Haagsma et al. (1982) and Ratnayake et al. (1988) have reported similar results for urea complexation experiments conducted for cod liver oil and sardine, respectively. The same way that Liu et al. (2006), who performed an optimization of concentration of PUFA n-3 of tuna oil by complexing with urea. Urea complexation under these conditions is highly efficient as it increased the total PUFA content from 35.75 to 97.62 (g/100 g TFA) (Table 1).
3.3. Purification of the structured triglyceride and analysis of the reaction products
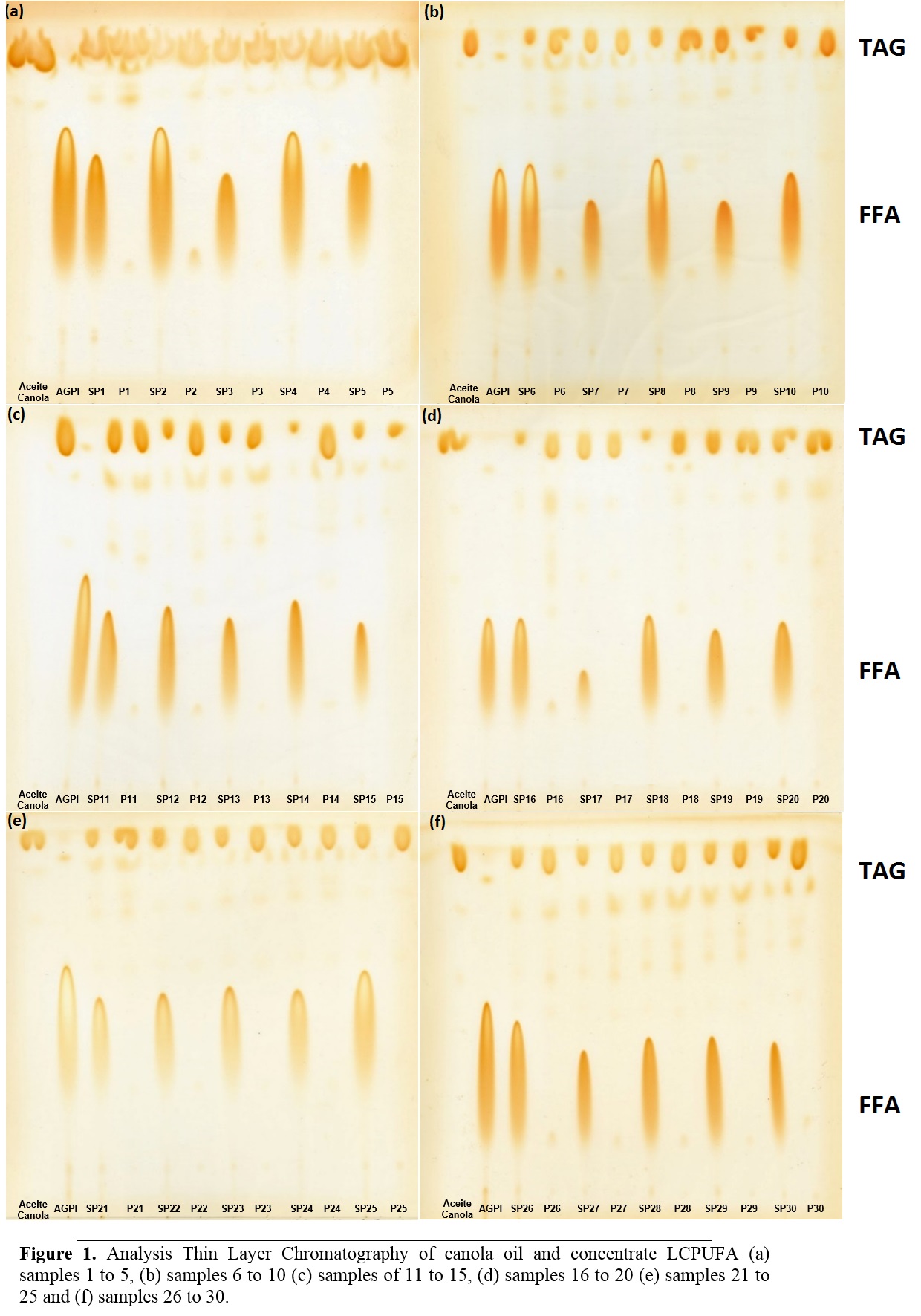
In Figure 1 the analysis Thin Layer Chromatography (TLC) of canola oil and concentrate LCPUFA (both upstream of enzymatic acidolysis) and samples without purification (WP) and Purified (P) (obtained after enzymatic acidolysis supercritical CO2 by the experimental design) is observed. In lane 1 TLC analysis (Figure 1: a, b, c, e, e and f) shows that canola contains only TAG, unlike the concentrate LCPUFA in lane 2 which presents only FFA. For other assays, for unpurified samples, the presence of both TAG and FFA is observed, unlike the purified samples show only TAG corresponding to the TAGs.
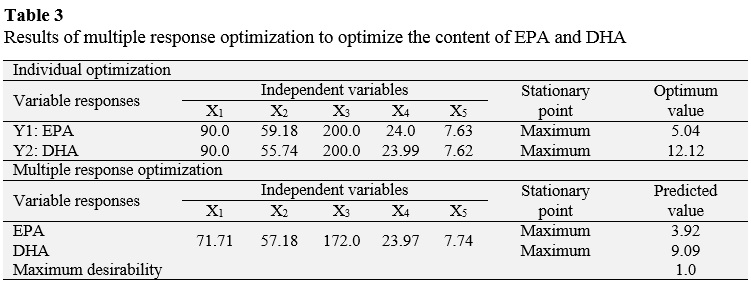
3.4. Optimization
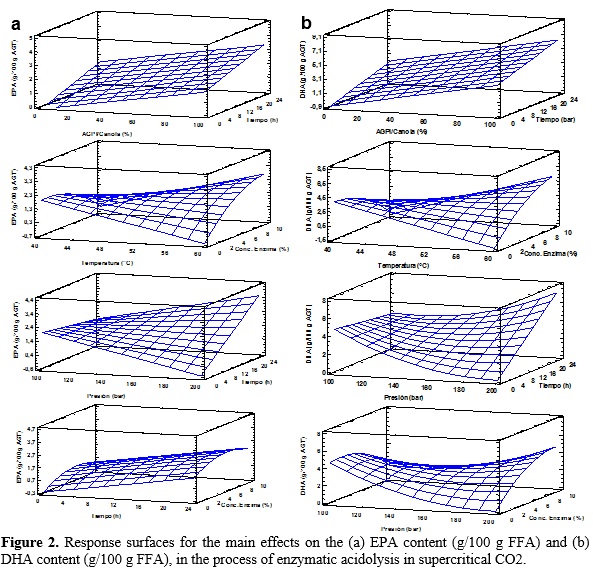
Table 2 shows the various combinations of the independent variables with their respective values of dependent variables. The relationship between independent variables and the dependent variables are seen as three-dimensional representations in response surface (Figure 2), in which the independent variables alternate in the axes to observe how the dependent variables influence: EPA and DHA content in the purified TAGs. From these results, it can be said that a proportion of 90.0 LCPUFA/canola oil, a reaction temperature of 59.18 °C, a reaction pressure of 200 bar, a reaction time of 24 h and concentration of enzyme 7.63% maximize the content of EPA in the purified TAGs to obtain an optimal value of 5.04 g/100 g FFA; while the maximum content of DHA in the purified TAGs (12.12 g/100 g TFA) is reached with a proportion of 90.0 LCPUFA/canola, 55.74 °C, 200 bar, 23.99 hours and 7.62% enzyme.
Furthermore, using the desirability function as multi-objective optimization tool, is obtained that both the EPA and DHA content in the purified maximizing TAGs (3.92 and 9.09 g/100 g FFA, respectively) when the combination is following: proportion of LCPUFA/canola oil 71.71%, temperature of 57.18 °C, pressure of 172.0 bar, time 23.97 h and concentration of 7.74% (Table 3).
Sharma et al. (2009) produce structured lipids containing a ratio of FA n-3 and n-6 1/1, incorporating FA n-3 (α-linolenic acid) flax oil in peanut oil using Lipozyme IM of Rhizomucor miehei, a reaction catalyzed by acidolysis in hexane. Optimal conditions for obtaining structured lipids were: enzyme concentration of 3.75% (w/w), temperature of 37.5 °C, incubation time 30.81 h and concentrated FFA ratio linseed oil/oil peanut 1.16 (w/w). Huang and Akoh (1996) used enzymatic hydrolysis to synthesize TAGs for modifying vegetable oils such as soybean oil with EPA, DHA and γ-linolenic.
In composition, canola oil does not present EPA and/or DHA fatty acids (Table 1); but after enzymatic acidolysis in supercritical carbon dioxide and canola oil concentrate LCPUFA, incorporation of EPA and DHA in the purified TAGs showed a content of EPA + DHA of 13.01 g/100 g TFA. This means that contains 13010 mg of EPA + DHA in 100 g of oil (or 130.1 mg per 1 g of oil). Thus, to meet the requirements of the FAO (2012) in EPA + DHA for adult men and women, 1.92 g of TAGs be needed to cover the 250 mg recommended. In the case of pregnant or breastfeeding women, 2.3 g of TAGs that would cover the 300 mg recommended.
The advantage of obtaining TAGs versus triacylglycerol (TAG), ethyl esters (EE) and free fatty acid (FFA) is that TAGs display greater bioavailability (effectively the human or animal body can use nutritionally AG n-3 of the marine oils and make a profit from them; Valenzuela and Sanhueza, 2009). Dyerberg et al. (2010) compared three concentrated preparations: TAGs, EE and FFA. The bioavailability of EPA + DHA of TAGs was higher (124%) compared to the natural fish oil (TAG), whereas the bioavailability of EE was lower (73%). The bioavailability of FFA (91%) did not differ significantly and had a mean bioavailability compared to TAG natural fish oil. Neubronner et al. (2011) performed supplementation (six months) of identical doses of EPA + DHA resulting in a greater increase in n-3 when consumed as TAGs when consumed as EE.
3.5. Characterization commercial salmon oil and canola oil
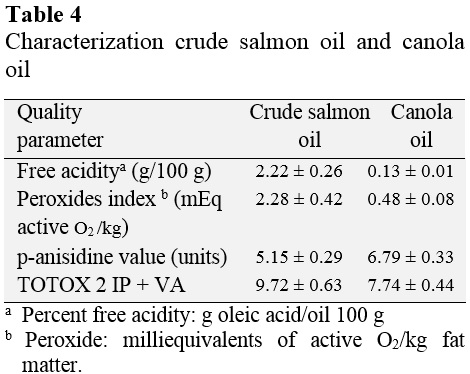
Table 4 presents the values of free acidity (g/100 g of oleic acid), peroxide value (mEq active oxygen/kg fat matter), p-anisidine value and TOTOX value for crude oil and salmon oil canola.
Results are expressed as arithmetic mean ± standard deviation (n = 3).
Canola oil has a free acidity value of oleic acid 0.13 g/100 g of oil and a peroxide value of 0.48 mEq active/kg fatty matter oxygen. These results are within the range set by the NTP 209 001: 1983 that allows up to 0.20% (0.20 g/100 g) of free acidity, expressed as oleic acid and peroxide value not greater than 5 mEq active oxygen/kg fatty substance.
The increase in the acid value indicates the degree of hydrolytic deterioration suffered the fat which is a measure of hydrolytic rancidity. In the case of FFA, typical values for raw material is between 0.5% to 5% and impaired fat between 0.5% to 1.5% (Masson, 1994).
Crude salmon oil has 2.22 g of oleic acid/100 g of oil as free acidity, 2.28 mEq active oxygen/kg fat as peroxide. The Global Organization EPA and DHA (CRN, 2015) sets a maximum peroxide value of 5 mEq/kg, which salmon crude oil is within the set value. Mendez et al. (2010) present values of free acidity and peroxide values for three oils of marine origin, whose peaks are in the order of 3.04 g/100 g of oil and 5.27 mEq active oxygen/kg fatty substance, respectively. These results corresponded to normal values of free acidity and peroxide values for raw fish oils.
Oxidative rancidity is a measure of the oxidation of polyunsaturated fatty acids TAG constituents. The peroxide value (PV) measured corresponding hydro-peroxides accumulated at the first sign of oxidative rancidity, i.e. primary oxidation (Masson, 1994).
With regard to the value of p-anisidine and TOTOX in some countries maximum values for these two parameters are set. The "International Fish Oil Standards" (IFOS, 2009) headquartered in Canada, indicates oil for human consumption for a maximum value of p-anisidine and TOTOX of 15 and 19.5, respectively. The "Council for Responsible Nutrition" (CRN, 2015), headquartered in the United States, according to a maximum value of p-anisidine and TOTOX of 20 and 26, respectively. Crude salmon oil has a p-anisidine value of 5.15 and TOTOX value of 9.71, values below the limits of the regulations mentioned, indicating acceptable values for this type of oil.
Pando et al. (2014) found values of p-anisidine, for crude oil and refined commercial salmon of 5.33 ± 0.03 and 5.14 ± 1.02, respectively. The p-anisidine value (VA) is a measure of the formation of secondary oxidation compounds highly reactive, predominantly carbonyl structures such as aldehydes and ketones (Masson, 1994).
4. Conclusions
The parameters of free acidity (2.22) (0.13), peroxide value (2.28) (0.48) p-anisidine value (5.15) (6.79) and TOTOX value (9 72) (7.74) realized the commercial salmon crude oil and canola oil, respectively, showed values within the ranges established by the NTP 209 002: 1983, IFOS (2009) and CRN (2015).
The parameters that maximize the content of EPA (3.92 g/100 g FFA) and DHA (9.09 g/100g TFA) in the purified structured triglycerides are: 71.71% of LCPUFA / Canola, 57.8 °C of temperature, 172.0 bar of pressure, 23.97 h and 7.74% of enzyme concentration. These values allow canola oil incorporating EPA and DHA from 0% to 13.01% in purified TAGs, making 1.92 g of TAGs cover the minimum daily 250 mg of EPA + DHA recommended by FAO (2012) for men and adult women and 2.3 g of TAGs cover the minimum daily 300 mg of EPA + DHA FAO (2012) recommended in the case of pregnant or breastfeeding women. Furthermore, the results show that the properties of the oil are improved because, when appearing TAGs it has greater bioavailability in the organism to TAG, EE and FFA.
5. References
AOCS. 1993. Official Methods and Recommended Practices of American Oil Chemists’ Society. 4th Ed. AOCSS Press, Champaign: Ca 5a-40:1, Cd 8b-90: 1 – 2, Cd 18-19: 1 – 2. [ Links ]
AOCS. 2009. Determination of cis-, trans-, saturated, mono-unsaturated, and polyunsaturated fatty acids in extracted fats by capillary GLC. AOCS Official Method Ce 1j-7. Sampling and analysis of commercial fats and oils. [ Links ]
Araya, J. 2008. Riesgos y beneficios del consumo de grasas y aceites. En Programa de Educación a Distancia. Departamento de Nutrición. Facultad de Medicina. Universidad de Chile. [ Links ]
Burr, M.L.; Fehily, A.M.; Gilbert, J.F.; Rogers, S.; Holliday, R.M.; Sweetnam, P.M.; Elwood, P.C.; Deadman, N.M. 1989. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 2(8666): 757- 761. [ Links ]
CRN (Council For Responsible Nutrition). 2015. Oxidation in Omega-3 Oils: An Overview. A White Paper Prepared by the Global Organization for EPA and DHA Omega-3s. Available in: http://crnusa.org/pdfs/GOED+CRNWhitePaper-Omega-3oxidation.pdf [ Links ]
Dyerberg, J.; Madsen, P.; Møller, J.M.; Aardestrup, I.; Schmidt, E.B. 2010. Biovailabity of marine n-3 fatty acid formulations. Prostaglandins, Leukotrienes and Essential Fatty Acids 83: 137-141. [ Links ]
FAO (Organización de las Naciones Unidas para la alimentación y la agricultura). 2012. Grasas y ácidos grasos en nutrición humana. Consulta de expertos. Estudio FAO Alimentación y Nutrición ISSN 1014-2916 FAO ISBN 978-92-5-3067336. FAO y FINUT, 2012 (edición española). [ Links ]
Ghazani, S.M.; Marangoni, A.G. 2013. Minor components in canola oil and effects of refining on these constituents: A review. Journal of the American Oil Chemists’ Society 90: 923 – 932. [ Links ]
Haggsma, N.; Van Gent, C.M.; Luten, J.B.; De Jong, R.W.; Van Doorn, E. 1982. Preparation of an ω-3 fatty acid concentrate from cod liver oil. Journal of the American Oil Chemists’ Society 59(3): 117 – 118. [ Links ]
Huang, K.H.; Akoh, C.C. 1996. Optimization and scale up of enzymatic synthesis of structure lipids using RSM. J Food Sci 6:137–41. [ Links ]
IFOS (International Fish Oil Standards). 2009. Consumer Report. Available in: http://www.nutrasource.ca/ifos/ [ Links ]
Iverson, J.L.; Weik, R.W. 1967. Correlation of fatty acid structure with preferential order of urea complex formation. Journal of the Association of Official Analytical Chemists’ 50: 1111 – 118. [ Links ]
Jiménez, M.J.; Esteban, L.; Robles, A.; Hita, E.; González, P.A.; Muñío, M.M.; Molina, E. 2010. Production of triacylglycerols rich in palmitic acid at sn-2 position by lipase-catalyzed acidolysis. Biochemical Engineering Journal 51: 172-179. [ Links ]
Liu, S.; Zhang, C.; Hong, P.; Ji, H. 2006. Concentration of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) of tuna oil by urea complexation: optimization of process parameters. Journal of Food Engineering 73: 203 – 209. [ Links ]
Masson, L. 1994. Criterio de calidad para materias grasas utilizadas frecuentemente en la nutrición animal y de peces. Available in: http://www.fao.org/docrep/field/003/ab482s/ab482s10.htm. [ Links ]
Masson, L.; Mella, M. 1985. Materias grasas de consumo habitual y potencial en Chile: Composición en ácidos grasos. 1ª ed, Editorial Universitaria, Santiago, Chile. 30 p. [ Links ]
Méndez, C.; Masson, L.; Jiménez, P. 2010. Estabilización de aceite de pescado por medio de antioxidantes naturales. A&G 30(3): 270 – 278.
Neubronner, J.; Schuchardt, J.P.; Kressel, G.; Merkel, M.; Von, Schacky C.; Hahn, A. 2011. Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. European Journal of Clinical Nutrition 65: 247-254. [ Links ]
Neuringer, M.; Connor, W.E.; Lin, D.S.; Barstad, L.; Luck, S. 1986. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc. Nat. Acad. Sci. 83: 4021-4025. [ Links ]
Osborn, H.T.; Akoh, C.C. 2002. Structured lipids-novel fats with medical, nutraceutical, and food applications. Comprehensive reviews in Food Science and Food Safety 45: 110-120. [ Links ]
Pando, M.E.; Bravo, B.; Berrios, M.; Galdames, A.; Rojas, C.; Romero, N.; Camilo, C.; Encina, C.; Rivera, M.; Rodriguez, A.; Aubourg, S. 2014. Concentrating n-3 fatty acids from crude and refined commercial salmon oil. Czech J. Food Sci 32(2): 169 – 176. [ Links ]
Ramírez, A. 2006. Salmon by-products proteins Circular área marina Nº 1027 FAO, Roma, Italia. [ Links ]
Ratnayake, W.M.N.; Olsson, B.; Matthews, D.; Ackman, R.G. 1988. Preparation of omega-3 PUFA concentrates from fish oils via urea complexation. Fat Science and Technology 90: 381 – 386. [ Links ]
Robles, A.; Esteban, L.; Giménez, A.; Camacho, B.; Ibañez, M.J.; Molina, E. 1999. Lipase-catalyzed esterification of glycerol and polyunsaturated fatty acids from fish and microalgae oils. Journal of Biotechnology 70: 379 – 391. [ Links ]
Robles, A.; Jiménez, M.J.; Esteban, L.; Gonzáles, P.A.; Martín, L.; Rodríguez, A.; Molina, E. 2011. Enzymatic production of human milk fat substitutes containing palmitic and docosahexaenoic acids at sn-2 position and oleic acid at sn-1,3 positions. LWT-Food Science and Technology 44: 1986-1992. [ Links ]
Sharma, M.; Rastogi, N.K.; Lokesk, B.R. 2009. Synthesis of structured lipid with balanced Omega-3: Omega-6 ratio by lipase-catalyzed acidolysis reaction: Optimization of reaction using response surface methodology. Process Biochemistry 44: 1284 – 1288. [ Links ]
Simopoulos, A.P. 2002. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56: 365 – 379. [ Links ]
Simopoulus, A. 1991. Omega-3 fatty acids in health and disease and in grown and development. Am. J. Clin. Nutr. 54: 438-468. [ Links ]
Strocchi, A.; Bonaga, G. 1975. Correlation between urea inclusion compounds and conformational structure of unsaturated C-18 fatty acid methyl esters. Chemical Physical Lipids 15: 87 – 94. [ Links ]
Uauy, R.; Valenzuela, A. 2000. Marine Oils: The health benefits of n-3 fatty acids. Nutrition 16: 680-684. [ Links ]
Uauy-Dagach, R.; Valenzuela, A. 1992. Marine oils as a source of Omega-3 fatty acids in the diet. Prog. Food Nutr. Sci. 16:199–243. [ Links ]
Valenzuela, A.; Sanhueza, J. 2009. Aceites de origen marino; Su importancia en la nutrición y en la ciencia de los alimentos. Revista Chilena de Nutrición 36 (3): 246 – 257. [ Links ]
Valenzuela, R.; Tapia, G.; González, M.; Valenzuela, A. 2011. Ácidos grasos omega-3 (EPA y DHA) y sus aplicaciones en diversas situaciones clínicas. Revista Chilena de Nutrición 38 (3): 356 – 367. [ Links ]
Wanasundara, U.A.; Shahidi, F. 1995. Storage stability of microencapsulated seal blubber oil. J. Food Lipids 2: 73 – 86. [ Links ]
Wanasundara, U.N. 1996. Marine oils: stabilization, structural characterization and omega-3 fatty acid concentration. Ph.D. thesis, Memorial University of Newfoundland, Canada. [ Links ]
Wanasundara, U.; Shahidi, F. 1999. Concentration of omega 3-polyunsaturated fatty acids of seal blubber oil by urea complexation: optimization of reaction conditions. Food Chemistry 65: 41-49. [ Links ]
* Autor para correspondência
E-mail: rsiche@unitru.edu.pe (R. Siche).
Recibido 08 julio 2015
Aceptado 06 noviembre 2015