Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Scientia Agropecuaria
versión impresa ISSN 2077-9917
Scientia Agropecuaria vol.7 no.3 Trujillo jul./set. 2016
http://dx.doi.org/10.17268/sci.agropecu.2016.03.18
ARTÍCULOS ORIGINALES
Compost and humic substance effects on soil parameters of Vitis vinifera L cv Thompson seedless
Paola Fincheira-Robles1,3; María Mercedes Martínez-Salgado1,2,*; Rodrigo Ortega-Blu1; Marc Janssens2
1 Grupo de Investigación en Suelo, Planta, Agua y Ambiente (GISPA). Universidad Técnica Federico Santa María. Santiago, Chile.
2 TROPENTropical Crops, Institute of Crop Science and Resource Conservation INRES Bonn Universität, Bonn Germany Auf dem Hugel 6, 53121 Bonn, PC. 531113.
3 Programa de Doctorado en Ciencias de Recursos Naturales. Universidad de La Frontera. Temuco, Chile.
Abstract
The use of organic amendments is common under the concept of integrated nutrient management (INM) in Vitis vinifera (Table grape) to improve plant and soil quality. The objective of this study was to evaluate compost (C) and humic substances (HS) mixed with mineral fertilizer (MF) in an INM program of V. vinifera cv Thompson seedless. The chemical, biochemical and microbiological parameters were evaluated in soil on 1-yearold V. vinifera plants growing on Alfisol soil. Five treatments and control were evaluated: (T1) C+MF, (T2) HS+MF, (T3) C, (T4) HS, (T5) MF and (T6) absolute control. The results indicated that the application of C and HS, increased β glucosidase and dehydrogenase activities, reaching values of 90.2 µg p-nitrophenol g1h-1 and 9.1 µg de TFP g-124h-1, respectively. In addition, pH was similar in all treatments while electrical conductivity increased with application of mineral and organic amendments, reaching 0.41dS m-1 in T2 (HS+MF). Furthermore, yeast concentration increased with organic amendments or mineral. Correlation analysis indicated significant and positive relationships between PO4-P concentration with MF (0.579) and C (0.431) and nitrogen with MF (0.868). These results support that INM, which combines mineral fertilization and organic amendments, improve positive changes in chemical soil properties and C cycling measured in terms of enzymatic activity in V. vinifera.
Key words: organic amendments, integral nutrition management, soil quality indicators.
1. Introduction
Nowadays, mineral fertilizers and organic amendments (OA) are commonly used as part of integrated nutrient management (INM) programs to increase productivity. The INM includes the use of good-quality organic matter sources and efficient mineral products in combination with good agricultural practices to get a good soil, plant and fruit quality, especially in grape orchards (Ortega, 2015).
Vitis vinifera L plantations in Chile cover more than 50 thousand ha of wine or table grape (Bravo, 2014). The nutrient requirements during the establishment period are very important to prevent phenotypic alterations and to improve plant and fruit quality. Normally, mineral fertilizers are used in combination with organic matter (goat manure or grape pomace compost) at rates of 10 a 15 t ha-1 to improve content of organic matter in soil, nutrients availability and beneficial microorganisms (Palma, 2006). In addition, they have the capacity to promote plant growth by increasing root biomass, cell rhizodeposition, root size and root hair density (Martínez et al., 2010a).
To follow the effects of the integrated nutrient management, soil quality indicators need to be defined, looking for sensitive chemical, biochemical, and microbiological properties and their correlation with soil productivity. Chemical properties include traditional chemical variables, as electrical conductivity, pH, total and available nitrogen (N), phosphorus (P) and potassium (K). Besides, biochemical parameters as enzymatic activity of dehydrogenase, phosphatases or β-glucosidase are used to determine the soil biological activity and the ability of organic complexes degradation for increasing nutrient availability through mineralization (Burns et al., 2013). Additionally, microbial variables are considered as soil quality indicators due to their direct relationship with mineralization and nutrients cycling (Sánchez et al., 2005). Until now, information about the effects of organic amendments application, such as compost and humic substances jointly with mineral fertilizer in soil of V. vinifera are unclear. This study hypothesized that IMN increases the biological activities and nutrient availability, and intended to evaluate IMN through chemical, biochemical and microbiological soil quality indicators on V. vinifera cv. Thompson seedless.
2. Materials and methods
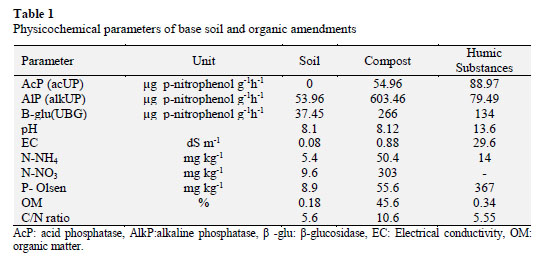
In this study 1-year old table grape (Vitis vinifera L) cv. Thompson seedless of commercial origin and grafted upon phenotypically similar rootstock was used. The plants were placed in 40 L pots under greenhouse conditions during summerautumn season; the mean air temperature was 25 °C. The base soil (anfisol) was obtained from an agricultural field located at coordinates 33° 7’1, 48’’S and 70° 48’ 57’’ W of the Metropolitan region characterized by low organic matter content (0.18%) and N-NO3 + N-NH4 (15 mg kg-1). The compost used was provided by "Agricola Bauza" and it was produced from grape pomace and stalks. The humic substance was obtained by alkaline extraction from mature compost (NNH4:N-NO3 < 3) (NCh 2880/04) (Table 1).
Finally, mineral fertilization was a combination of 20 g of (NH4)2SO4 with the nitrification inhibitor 3,4 DMPP (Novatec Solub 21), 150 mL 1% H3PO4 and 10 g of K2SO4 for each liter of solution.
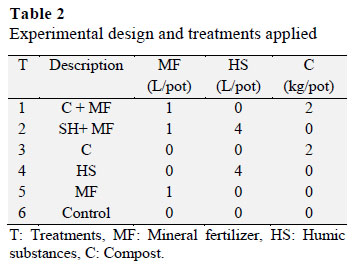
Five treatments were evaluated against an absolute control in a completely rando mized design with four replicates for each treatment (Table 2). The compost was incorporated at a rate of 2 kg per pot, and humic substances at 4 L pot-1.
The pH and EC were measured according to Sadzawka (1990). The Ammonia (N NH4) and nitrate (N-NO3) contents were quantified by colorimetric method des cribed by Mulvaney (1996) and phosphorus availability was determined accor ding to Sadzawka et al. (2006).
Enzymatic activities were measured, using ρ-nitrophenyl method at pH 6.5 and 11 for acid and alkaline phosphatases, respectively. In addition, ρ-nitrophenylβ-D glucopironoside (PNG) was used for βglucosidase activity (García-Ruiz et al., 2008) and 2,3,5-triphenyltetrazolium chloride (TTC 3%) to determine dehydro genase activity (Acosta and Paolini, 2005). Furthermore, the concentration of micro organisms was determined using plate counting technique in milk and starch agar for proteolytic and amylolytic micro organisms, respectively. Potato dextrose agar (Difco) was used to determine total yeast (Martínez et al., 2010b).
Variables were measured 11 weeks after the planting and subjected to normality test before analysis of variance. The normalized data was compared by ANOVA through Tukey test (p < 0.05). Pearson correlation was performed among measu red variables. Data analyses were made using the software Statistix, version 10.0.
3. Results and discussion
Vitis vinifera cv. Thompson seedless is cultivated in a wide range of soils and climates, requiring a proper nutrient application to allow normal growth and development of plants (Palma, 2006). Moreover, the measurements of soil quality indicators give important information about the effects of organic amendments and mineral fertilizers on soil.
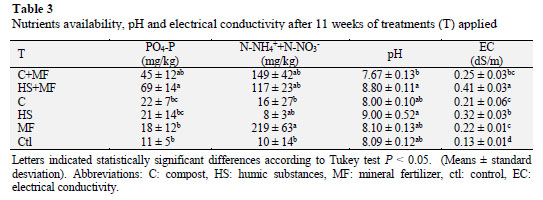
The results corresponding to chemical properties (Table 3) indicated that EC and pH are variables that differentiate treat ments applied.
In this study, both parameters were higher in treatments with HS compared with compost and mineral fertilizer. In addition, compost showed a pH buffer activity in contrast to HS, when both are applied with mineral fertilizer. These results are in agreement with a study carried out by Mijangos et al. (2006) which showed that pH is higher in treatments with organic amendments (pH 5.9) compared to the mineral fertilizer treatments (pH 5.35.4).
Additionally, phosphorus increased in treatments with mineral fertilizer in conjunction with both organic amendments, reaching 46 and 70 mg kg-1 of PO4-P with compost and humic substances, respectively. The compost and humic substances provided a similar amount of phosphorus after treatment application (p < 0.05), while mineral fertilizer delivered lower concentrations of available phosphorus, reaching a concentration of 18 mg kg-1 of PO4-P.
The results showed that an important amount of P availability is contributed by the application of both organic amendments and mineral fertilizer together, due to the fact that these treatments include a direct source of P constituted by H3PO4 and organic mineralized P as P-PO4, probably as a result of phosphatases activity and organic acids production that allow hydrolysis or solubilization of organic phosphorus (Sharma et al., 2011).
Furthermore, Saha et al. (2008) showed that phosphatase activity affects positively the availability of P when organic amendments are applied at high rates. This larger availability is directly relates with phosphatase activity, which in turns could be associated to phosphorus solubilizing bacteria. This functional group plays an important nutrition role by enhancing P availability releasing from organic soil P pools by solubilization and mineralization. Moreover, humic substances supply phosphorus, reaching 367 mg kg-1. On the other hand, mineral fertilizer increases nutrients for microbial pool so increase biological activity.
Nitrogen availability showed a higher concentration with mineral fertilizer application, reaching 220 mg kg-1 of N. Moreover, the treatments that included MF and organic amendments (149 and 117 mg kg-1 N) tended to increase the amount of soluble nitrogen compared with C (16 mg kg-1 of N) and HS (9 mg kg-1 of N). This effect is due to the slow mineralization of nitrogen from organic sources. A study performed by Flavel et al. (2005) indicated that organic amendments have a slow nitrogen mineralization because the compost from grape marc causes immobilization of N during 50 days. These results indicate that organic amendments and MF are an important source of phosphorus availability whereas only MF is able to provide nitrogen on Vitis vinifera cv. Thompson seedless.
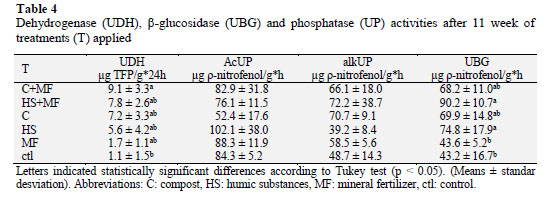
Nutrient availability can depend on biochemical factors that determine the hydrolytic activity of complex organic polymers present in the soil. Table 4 shows that dehydrogenase and β-glucosidase activities have a strong relationship with nutrient cycling and relevance as reliable indicators that reflect microbial activity.
The dehydrogenase is an intracellular enzyme involved in the oxidation-reduc tion processes associated with microbial metabolism, whereby its activity depends on the presence of physiologically active microorganisms (Makoi and Ndakidemi, 2008).
In this study dehydrogenase activity increased with the application of organic amendments, reaching highest values in the treatments related to compost and mineral fertilizers probably due to high microbial load and appropriate nutrient source that allow keeping microorganisms physiologically active, and where mineral fertilizers can act as additional nutritional source. Romero et al. (2010) reported that the application of organic matter from grape pomace increases dehydrogenase enzyme activity in soils with low organic carbon content. Similarly, Adak et al. (2014) indicated that this enzyme increase with vermicompost addition.
In addition, phosphatase activity has been reported to play an important role in the cycling of phosphorus and it is described as a responsible enzyme for hydrolyzing organic phosphorus (Crecchio et al. 2004; Martínez et al., 2010a). In this study no statistically significant differences among treatments were found, but both acid and alkaline phosphatase activities showed a trend to increase with the applied treatments. Finally, β-glucosidase is an extracellular enzyme that catalyzes the final step of the cellulose degradation. In this study, its activity increased significantly with the application of organic amendments and decreased with higher rates of chemical fertilizers. Humic substances stimulated this activity probably due to its ability of adsorbing β-glucosidase through soil complexes and the presence of microorganisms. Furthermore, Bastida et al. (2012), demonstrated that humic substances protected soil enzymes and hence, the humus-enzyme complexes from microbial attack. Allison and Vitouseh (2005) indicated that β glucosidase increased in the soil with higher amounts of carbon and nitrogen though application of organic and inorganic sources.
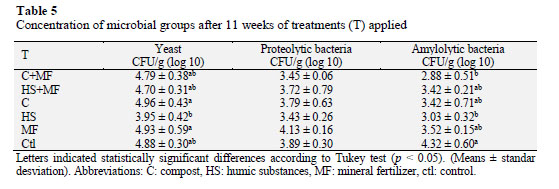
Regarding microbial functional groups (Table 5), these have the ability of producing enzymes that degrade the complex organic compounds found in the soil, which in turn enhances nutrient availability for plant roots.
Results indicated that yeast increased with compost or mineral fertilizer application, probably due to the fact that both amendments can act as direct and indirect nutrient sources. However, no significant differences (p < 0.05) were found in terms of proteolytic bacteria, probably due to the low concentration of substrate or the nitrogen availability from organic or inorganic sources.
4. Conclusions
The results demonstrated that soil quality indicators allow determining the effects of Integrated Nutrient Management. The β glucosidase activity, dehydrogenase activity, nutrient concentrations, yeast and amylolytic bacteria were sensitive parameters and good indicators of soil quality in V. vinifera.
Acknowledgements
The authors would like to acknowledge Project FONDECYT 1130975 and Projects USM 28.09.49 and 28.11.69 for partially funding this research. Special thanks are given to Empresas Bauzá, particularly to Mr. Walter Cortés, for helping during the experimental part of this research.
References
Adak, T.; Singha, A.; Kumar, K.; Shukla, S.; Singh, A.; KumarSingh, V. 2014. Soil organic carbon, dehydrogenase activity, nutrient availability and leaf nutrient content as affected by organic and inorganic source of nutrient in mango orchard soil. Journal of Soil Science and Plant Nutrition 14: 394-406. [ Links ]
Acosta, Y.; Paolioni, J. 2005. Actividad de la enzima deshidrogenasa en un suelo calciorthids enmendado con residuos orgánicos. Agronomía Tropical 55: 217-232. [ Links ]
Allison, S.; Vitousek, P. 2005. Response of extracellular enzymes to simple and complex nutrients input. Soil Biology and Biochemistry 37: 937-944. [ Links ]
Bastida, F.; Moreno, J.; Hernández, T.; García, C. 2012. Effects of organic amendments on soil carbon fractions, enzyme activity and humus–enzyme complexes under semi-arid conditions. Ecohydrology >and Hydrobiology 53: 94-102. [ Links ]
Bravo, J. 2014. Boletín frutícola: Avance Julio 2014. Oficina de Estudios y Política Agraria (ODEPA). Gobierno de Chile. Santiago, Chile. [ Links ]
Burns, R.; de Fores, J.; Marxsen, J.; Sinsabaugh, R.; Stromberger, M.; Wallensteinf, M.; Weintraub, M.; Zoppini, A. 2013. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biology and Biochemistry 58: 216-234. [ Links ]
Crecchio, C.; Curci, M.; Pizzigallo, M.; Ricciuti, P.; Ruggiero, P. 2004. Effects of municipal solid waste compost amendments on soil enzyme activities and bacterial genetic diversity. Soil Biology and Biochemistry 36: 1595-1605. [ Links ]
Flavel, T.; Murphy, D.; Fillery, D. 2005. Gross N mineralization rates after application of composted grape marc to soil. Soil Biology and Biochemistry 37: 1397-1400. [ Links ]
García-Ruiz, R.; Ochoa, V.; Hinojosa, B.; Carreira, J. 2008. Suitability of enzyme activities for the monitoring of soil quality improvement in organic agricultural systems. Soil Biology and Biochemistry 40: 2137-2145. [ Links ]
Makoi, J.; Ndakidemi, P. 2008. Selected soil enzymes: Examples of their potential roles in the ecosystem. African Journal of Biotechnology. 7: 181-191. [ Links ]
Martínez, M.; Gutiérrez, V.; Novo, R. 2010a. Micro biología aplicada al manejo sustentable de suelos y cultivos. Editorial USM. Santiago, Chile. [ Links ]
Martínez, M.; Pedroza, A.; Gutiérrez, V. 2010b. Métodos microbiológicos, físicos y químicos con aplicación ambiental. Editorial USM. Santiago, Chile. [ Links ]
Mijangos, I.; Pérez, R.; Albizu, I.; Garbisu, C. 2006. Effects of fertilization and tillage on soil biological parameters. Enzyme and Microbial Technology 40: 100-106. [ Links ]
Mulvaney, R. 1996. Methods of Soil Analysis: Chemical Methods. Soil Science Society of America. Madison, United States. [ Links ]
Ortega, R. 2015. Integrated nutrient management in conventional intensive horticulture production systems. Acta Horticulturae 1076:159-164. [ Links ]
Palma, J. 2006. Estrategia de fertilización en vid de mesa diseños y monitoreos. Guía de manejo nutrición vegetal de especialidad: Uva. Soquimich. Santiago Chile. [ Links ]
Romero, E.; Fernández-Bayo.; Castillo, J.; Nogales, R. 2010. Enzyme activities and diuron persistence in soil amended with vermicompost derived from spent grape marc and treated with urea. Applied Soil Ecology 44:198-204. [ Links ]
Sadzawka, A. 1990. Métodos de análisis de suelos. Instituto de Investigaciones AgropecuariasINIA. Santiago, Chile. [ Links ]
Sadzawka, A.; Carrasco, M.; Grez, R.; Mora, M.; Flores, H.; Neaman, A. 2006. Métodos de análisis recomendados para los suelos de Chile Revisión. Instituto de Investigaciones AgropecuariasINIA. Santiago-Chile. [ Links ]
Saha, S.; Mina, B.; Gopinath, K.; Kundu, S.; Gupta, A. 2008. Relative changes in phosphatase activities as influenced by source and application rate of organic composts in field crops. Bioresource Technology 99: 1750-1757. [ Links ]
Sánchez, C.; Mejía, C.; Figueroa, C.; Esquivia, M.; Agudelo, L.; Zapata, N.; Gómez, M. 2005. Estudio de cepas nativas amilolíticas. Red de Revistas Científicas de América Latina, el Caribe, España y Portugal 12: 21-28. [ Links ]
Sharma, S.; Kumar, V.; Tripathi, R. 2011. Isolation of phosphate solubilizing microorganism (PSMs) from Soil. Journal of Microbiology and Biotechnology Research 1: 90-95. [ Links ]
* Corresponding author
E-mail: maria.martinez@usm.cl (M.M. Martinez-Salgado).
Received March 30, 2016.
Accepted Jun 30, 2016.