Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Scientia Agropecuaria
versión impresa ISSN 2077-9917
Scientia Agropecuaria vol.9 no.3 Trujillo jul./set. 2018
http://dx.doi.org/10.17268/sci.agropecu.2018.03.12
ORIGINAL ARTICLES
The effect of the ethanol extract from the Dracontium spruceanum rhizome on hematologic and biochemical profiles and performance parameters of broiler chickens
Daniel Paredes-López*; Rizal Robles-Huaynate; Darío Mendoza-Isla; Clarita Mendoza-Pérez; Hugo Saavedra-Rodríguez
Departamento de Ciencia Animal, Universidad Nacional Agraria de la Selva, Carretera Central Km 1.21, Po Box 156, Tingo María, Peru.
Abstract
The objective of the research was to evaluate the antioxidant capacity and effect of the ethanol extract from the Dracontium spruceanum rhizome (EERDs) on the blood, biochemical and productive parameters of chickens. To do so, ninety, male, Cobb 500 broiler chickens were used. Once the dehydrated extract was obtained, it was place in the drinking water at concentrations of 0.0, 0.35 and 0.70 mg/mL of EERDs. The birds were distributed into three treatments, five repetitions and each repetition had six chickens. The variance analysis was done with the statistical program InfoStat and the averages of the treatments were analyzed with the 5% Tukey test. The results showed that chickens that consumed drinking water with EERDs presented (p < 0.05) greater concentrations of hematocrit, hemoglobin and erythrocytes; meanwhile, the serum protein and the glucose did not change (p > 0.05). The daily food consumption diminished (p < 0.05) and the DWG and FRC were not influenced (p > 0.05) by the consumption of EERDs in the drinking water. It is concluded that the consumption of EERDs by broiler chickens from 1 to 35 days of age produces greater concentrations of the levels of red blood cells and diminishes the feed intake.
Keywords: Dracontium spruceanum; hematocrit; productive performance; antioxidant capacity.
1. Introduction
The intensive production of domestic animals, conditions them to stressful oxidative processes, due to the handling conditions such as the confinement, the breeding density, productive stage, etc. (Huerta-Jimenez et al., 2005; Koknaroglu and Akunal, 2013).
Oxidative stress occurs in all animal species, above all, in those with early growth, like the broiler chicken, which due to its physiological characteristics and intense metabolic activity, is permanently producing oxidative substances (DrÖge, 2002). To overcome this demand, the first line of action of the organism is to resort to its endogenous antioxidant reserves and the use of nutrients to alleviate the negative effects.
To improve the adverse effects of oxidative stress and the productive indices, traditionally, we resort to the use of pharmaceuticals which, on one hand, develop microorganism’s resistant to the drugs and on the other hand, generate residuals in the derived products that could have collateral effects for the consumer (Bistoletti et. al., 2011; Sakai et al., 2016). Due to this, at present the industry is introducing natural antioxidants to eliminate the factors that negatively affect production (Jahanian and Mirfendereski, 2015; Boostani et al., 2015; Gerasopoulos et al., 2015; Soltani et al., 2016).
Dracontium spruceanum is a plant in the Peruvian Amazon which is characterized by its antioxidant and immunomodulatory properties (Giovannini and Howes 2017; Benavides et al., 2009; Napolitano et al., 2011; Lovera et al., 2006); due to this, its use in the feeding of broiler chickens could improve their well-being and health. The objective was to determine the effect of the ethanol extract from the Dracontium spruceanum rhizome in the well-being and health of broiler chickens under an intensive rearing system, through the evaluation of the in vitro antioxidant activity, blood profiles and productive parameters.
2. Materials and methods
Obtaining the Ethanol Extract from the Dracontium spruceanum Rhizome
For this, a 200 g sample of the rhizome, dried and crushed, was placed in a jar, later ethanol was added at 75% to reach one liter and it was left macerating for fortyeight hours. Later, the suspension was stirred and filtered with cotton. The filtered substance, containing the extract, was submitted to evaporation of the ethanol, using a rota vapor and finally, finished drying in an oven at 65 °C. The ethanol extract obtained was packaged and stored.
Determining the antioxidant activity in vitro
The antioxidant capacity of the ethanolic extract from D. spruceanum, was evaluated using the method of sequestering the free radical 1,1 diphenil-2-picrylhidrazyl (DPPH) (100 uM) (Brand-Williams et al., 1994; Sánchez-Moreno, 2002), causing a reaction with 25, 75, 125, 175 and 250 µg/mL of EERDs; the readings were done in a UV light spectrophotometer and Electron Corporation visual, Genesys-6 model, with a 515 nm filter, every thirty seconds for a total time of ten minutes.
The percentage of inhibition of the DPPH obtained by each of the concentrations was used to determine the Inhibition Coefficient (IC50), which was expressed in µg/mL, indicating the necessary quantity of the aqueous atomized extract from D. spruceanum to inhibit, at 50%, the DPPH radical.
Experimental Animals
The work was done with ninety baby male chickens from the Cobb Vantress 500 breed, with an average weight of 46.11 ± 1.6 g; which were distributed into three treatments, with five repetitions and each repetition with six birds, which were bred under similar handling and feeding conditions. Three balanced diets were formulated, the composition of which was according to the requirements of the breed (Rostagno et al., 2011).
Design and Statistical Analysis
The hematological profiles were submitted to a completely randomized design CRD with a factorial arrangement of 3 x 2 + 1 (three levels of EERDs x 2 ages + 1 control). The productive performance parameters were submitted to a completely randomized design (CRD) with three treatments, five repetitions and each repetition with six chickens. The variance analyses were done with the statistical program InfoStat (Universidad Nacional de Cordova, 2016) and the averages were compared using the Tukey test (5%).
3. Results and discussion
Antioxidant Activity of the Ethanol Extract from the Dracontium spruceanum Rhizome
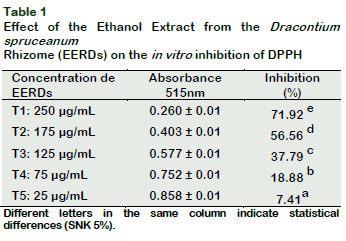
The inhibition capacity was related to the concentration of EERDs, with T1 (250 µg/mL) showing the greatest free radical inhibition capacity (p < 0.05) (Table 1).
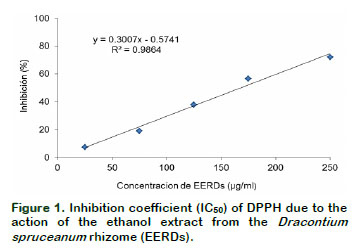
By the linear equation, it was determined that he concentration of EERDs required to inhibit the DPPH at 50% was 164.37 µg/mL (Figure 1). The treatments with 25 µg/mL and 250 µg/mL generate an inhibition of 7.41 and 71.92%, respectively; showing that the greater the concentration of EERDs, the greater the inhibition of the DPPH radical. Velandia (2009), did a test on the antioxidant activities, with fraction and extracts from Dracontium croatti, at different concentrations (1-100 μg/ml), with butanol, on the retention effect of the DPPH; obtaining an IC50 with 25.12 μg/ml of the extract. This difference could be associated to the specie of Dracontium. The antioxidant activity of Dracontium spruceanum could be associated to the presence of highly unsaturated nuclei such as polyphenols, flavonoids and quinones (Rivera, 2012), as well as to the presence of aromatic compounds like sterols, triterpenoids and alkaloids (Rengifo, 2007). In a similar study with an aqueous atomized extract from Uncaria tomentosa, it was found that the inhibition of the DPPH radical was 88.17% with 250 µg/ml of the extract (Sandoval, 2012).
Hematological and Biochemical Profiles
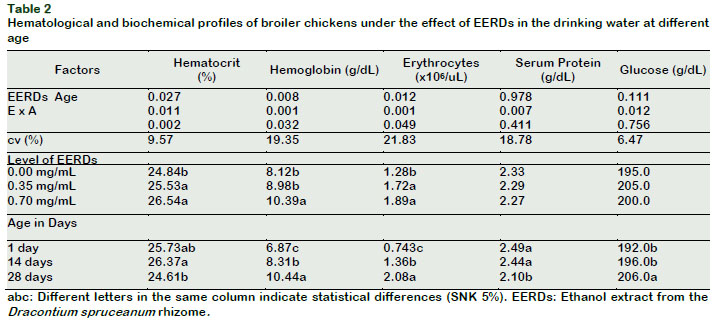
The hematocrit, hemoglobin and total erythrocyte profiles of the experimental chickens increased as the level of EERDs in the drinking water (p < 0.05) increased. However, the serum protein and glucose profiles were similar between treatments (p > 0.05) (Table 2).
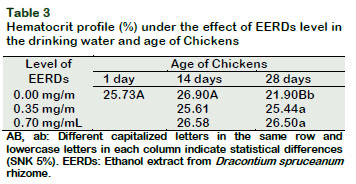
The hematocrit increased at twenty-eight days old (p < 0.05) as the level of the EERDs increased in the drinking water; these results resemble the study by Sandoval (2012), who reported a progressive increase in the percentage of hematocrit in broiler chickens every time the IC50 from the aqueous atomized extract of catâs claw was increased in the drinking water.
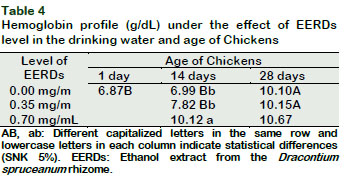
The level of hemoglobin increased at fourteen days of age (p < 0.05) as the levels of EERDs increased in the drinking water (Tables 2 and 4). This also increased with the increase in the birds age from the control group and in the group with 0.35 mg/mL of EERDs (p < 0.05). These results are similar to those reported by Sandoval (2012), meanwhile, Reátegui-Inga et al., (2012) reported an increase in hemoglobin at forty-eight days old.
This profile also increased as an effect of the age of chickens up to fourteen days only in the control group, (p < 0.05) (Tables 2 and 3). This could be associated to the fact that the antioxidant effect of the EERDs on the cells allows for a lengthening of the life of the erythrocytes, covering up the effect of age on erythrocytes life span in the treatment groups chickens; contrasting with the physiological patterns which are characterized by a progressive increase in the level of hematocrit each time that the chickens increase in age (Vásquez et al., 2012).
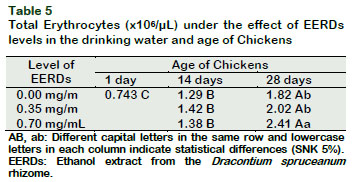
The number of erythrocytes in broiler chickens increased (p < 0.05) as the dose of the EERDs in the drinking water increased at 28 days (Tables 2 and 5). The level of erythrocytes also increased with the increase of the age, as much in the control group as in the groups with 0.35 and 0.70 mg/mL of EERDs (p < 0.05).
The level of hematocrit, hemoglobin and the number of erythrocytes of broiler chickens are within the normal values (Reece, 2015). The increase in hemoglobin, hematocrit and total erythrocytes as the EERDs increases may be related with those described for Uncaria tomentosa. The numerous groups of compounds present in this plant have protective effects on erythrocytes and diminish the levels of hemoglobin oxidation and the lipid peroxidation; as well as lowering the levels of ROS and hemolysis provoked by 2-4 dichlorophenol in human erythrocytes (Bors et al., 2011; Bucowska et al., 2012).
The polyphenols, principal components of the Uncaria tomentosa extracts, could act, not just sequestrating free radicals and inhibitors of lipid peroxidation, but also have the capacity to interact directly with biological membranes, causing them to be more resistant to oxidative alterations (Dreifus et al., 2010). These same mechanisms permit human erythrocytes to induce an increase in membrane thickness, followed by an increase in size and morphological variation (Bors et al., 2012). In the present study, it was found that an interaction exists, between the dose of EERDs in the drinking water and the age of chickens, on the levels of hematocrit, hemoglobin and erythrocytes (p < 0.05) (Tables 2, 3, 4 and 5). The results show that the levels of hematocrit, hemoglobin and total erythrocytes, in general, increase with the age increase and the increase in the level of EERDs, which could be associated to physiological mechanisms of increase in oxygen demand by the muscular mass for metabolism (Skovgaard et al., 2010) and antioxidant and immunomodulatory activity of Dracontium (Giovannini and Howes, 2017; Benavides et al., 2009; Napolitano et al., 2011), similar to those produced by U. tomentosa (Vielma, et al., 2014; Wagner, et al., 1985).
The age of chickens influenced (p < 0.05) the levels of serum protein and glucose, denoting that chickens at one and fourteen-day old presented a greater concentration of serum protein (p < 0.05) than the chickens at twenty-eight days old (Table 2). These results contrast with serum protein levels produced by the physiological mechanisms (Eckersall, 2008); different results from those were reported by Sandoval (2012). On the contrary, the glucose resulted at a greater level in the chickens of twenty-eight days old than those at one and fourteen days old (p < 0.05). This data contrasts the results of Gonzáles et al. (2001), who showed a lowering of the serum glucose level in broiler chickens as age increased.
Productive Parameters
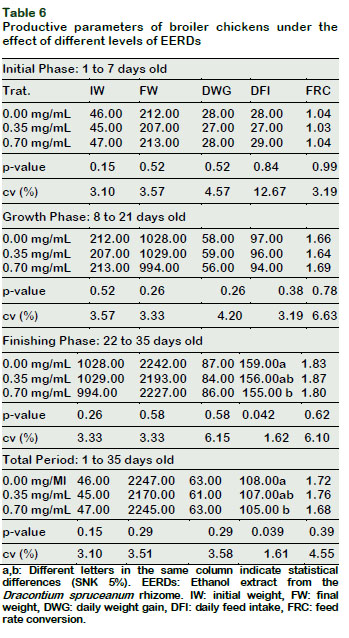
The daily feed intake (DFI) diminished as a result of the EERDs increase in the drinking water (p < 0.05) during the finishing phase (22-35 days) and during the total rearing phase (1-35 days) (Table 6). However, the daily weight gain (DWG) and the feed rate conversion (FRC) of the male broiler chickens during the initial, growth, and finishing phases and the total period, were not influenced by the level of EERDs in the different productive phases of the broiler chickens (p > 0.05).
These results could be correlated to the toxic effects of certain plants with similar nutraceutical characteristics to Dracontium, as is the case of U. tomentosa, for which toxic effects have been found in rats, mice and fish (Méndez et al., 2014; Ibrahim et al., 2009; Cala and Kochenborger, 2015), and associated with the initial phase of chickens (Sandoval, 2012).
Notwithstanding, the lack of effect of the DFI lowering on the weight gain of chickens could also be attributed to the fact that Dracontium possesses similar characteristics to those reported for U. Tomentosa, which improve the length of the intestinal villi and as a result the improvement in the absorption of nutrients (Yunis-Aguinaga et al., 2015); thus, counteracting the potential toxic effects attributed, in the same manner, to this plant.
4. Conclusions
The ethanol extract of the Dracontium spruceanum rhizome at a concentration of 164.37 ug/mL inhibited 50% the DPPH; at the same time, the levels of hematocrit, hemoglobin and total erythrocytes increased, while the daily food consumption decreased and there was no effect on the daily weight gain and food rate conversion in the broilers. Other research to clarify potential toxic effects and promotors of intestinal health from D. spruceanum should be done.
References
Benavides, A.; Napolitano, A.; Bassarello, C.; Carbone, V.; Gazzerro, P.; Malfitano, A.; Saggese, P.; Bifulco, M.; Piacente, S.; Pizza, C. 2009. Oxylipins from Dracontium loretense. J. Nat. Prod. 72 (5): 813–817. [ Links ]
Bistoletti, M.; Moreno, L.; Alvarez, L.; Lanusse, C. 2011. Multiresidue HPLC method to measure benzimidazole anthelmintics in plasma and egg from laying hens. Evaluation of albendazole metabolites residue profiles. Food Chemistry 126: 793-800. [ Links ]
Boostani, A.; Sadegui, A.; Mousavi, S.N.; Chamani, M.; Kashan, N. 2015. Effect of organic, inorganic and nano-Se on growth perfomance, antioxidant capacity, cellular and humoral immune response in broiler chicken exposed to oxidative stress. Livestock science 178: 330-336 [ Links ]
Bors, M.; Cisinska, P.; Michalowicz, J.; Wieteska, P.; Gulewicz, K.; Bucouska, B. 2012. Evaluation of the effect of Uncaria tomentosa extract on the size and shape of human erythrocyte (in vitro). Environmental toxicology and pharmacology 233: 127-134. [ Links ]
Brand–Williams, W.; Berset, C.; Cuvelier, M.E. 1994. Use of free radical method to evaluate antioxidant activity. J. Agric. Food Chem. 1234–1238. [ Links ]
Bucowska, B.; Bors, M.; Gulewicz, K.; Koter-Michalak, M. 2012. Uncaria tomentosa extracts protects human erythrocytes catalase against induced by 2, 4-D-Na and its metabolites. Journal of Etnopharmacology 50: 2123-2127. [ Links ]
Cala, D.; Kochenborger, J. 2015. Suplementacao de una-de-gato (Uncaria tomentosa) en dietas paratilapias-do-Nilo e acará-bandeira (Pterophyllum scalare). Tesis posgrado. Sao paulo. Universidad Estadual Paulista. [ Links ]
Dreifus, A.; Bastos-Pereira, A.; Avila, T.; Da Silva, B.; Rivero, A.; Aguilar, J.; Acco, A. 2010. Antitumoral and antioxidant effects of a hidroalcoholic extract of cat’s claw (Uncaria tomentosa) (Willd Ex Roem & Schult) in an in vivo carcinoma sarcoma model. Journal of Etnopharmacology 130(1): 127-133.
DrÖge, W. 2002. Free radicals in the physiological control of cell function. Physiol. 82: 47-95. [ Links ]
Eckersall, P. 2008. Chapter 5: Proteins, proteomics and dysproteinemias. In: Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemsitry of Domestic Animals. 6th Edition, Academic Press. P. 117-155. [ Links ]
Gerasopoulos, K.; Stagos, D.; Kokkas, S.; Petrotos, K.; Kantas, D.; Goulas, P.; Kouretas, D. 2015. Feed supplement with byproducts from olive oil mil wastewater processing increases antioxidant capacity in broilers chicken. Food and Chemical Toxicology 82: 42-49. [ Links ]
Giovannini, P., Howes, M. 2017. Medicinal plants used to treat snakebite in Central America: Review and assessment of scientific evidence. Journal of Ethnopharmacology 199: 240-256. [ Links ]
Gonzáles, F.; Haida, K.; Mahl, D.; Giannesi, G.; Kronbauer, E. 2001. Incidencia de doencas matabólicas em frangos de corte no sul do brasil e uso do perfil bioquímico sanguíneo para seu estudo. Rev. Bras. Cienc. Avic. 3(2): 52-63. [ Links ]
Huerta-Jimenez, M.; Ortega-Cerrilla, M.; Cobos-Peralta, M.; Herrera-Jaro, J.; Diaz-Cruz, A.; Guinzberg-Perrusquia, R. 2005. Stress oxidativo y el uso de antioxidantes en animales domésticos. Interciencia 30(12): 728-734. [ Links ]
Ibrahim, K.; Al-Ashban; Al-Sammani, S.A. 2009. A study of the toxicity of cat´s claw herbal medicine. Research Journal of Pharmacology 3(3): 52-57
Jahanian, R.; Mirfendereski, E. 2015. Effect of high stocking density on performance, egg quality, and plasma and yolk antioxidant capacity in laying hens supplemented with organic chromium and vitamin C. Livestock Science 177: 117-124. [ Links ]
Koknaroglu, H.; Akunal, T. 2013. Animal welfare: An animal science approach. Meat Science 95: 821-827 Lovera, [ Links ] A.; Bonilla, C.; Hidalgo, J. 2006. Efecto neutralizador del extracto acuoso de Dracontium loretense (Jergón Sacha) sobre la actividad letal del veneno de Bothrops atrox. Rev. Perú Med. Exp. Salud Pública 23(3): 177-181. [ Links ]
Méndez, P.; Ponce, F.; Fraga, D.; Pípole, F.; Perazzo, F.; Hueza, I. 2014. High doses of Uncaria tomentosa (cat’s claw) reduces blood glucose levels in rats. Int. J. Pharm and pharm Science 6(2): 410-415.
Napolitano, A.; Benavides, A.; Pizza, C.; Piacente, S. 2011. Qualitative on-line profiling of ceramides and cerebrosides by high performance liquid chromatography coupled with electrospray ionization ion trap tandem mass spectrometry: the case of Dracontium loretense. J. Pharm. Biomed. Anal. 55(1): 23–30. [ Links ]
Reece, W. 2015. The composition and functions of blood. In: Reece, W.O.; Erickson, H.H.; Goff, J.P.; Uemura, E.E. Duckes’ Physiology of Domestic Animals 13th Edition, Wiley Blackwell, OX, UK. P. 114-136.
Reátegui-Inga, R.; Paredes-López, D.; Robles-Huaynate, R. 2012. Efecto de diferentes niveles de torta de sacha inchi (Plukenetia volúbilis) sobre el hígado y el perfil bioquímico sanguíneo de pollos de carne. Folia Amazónica 24(2): 131-138. [ Links ]
Rengifo, E. 2007. Las ramas floridas del bosque "Experiencias en el manejo de plantas medicinales amazónicas". Instituto de Investigaciones de la Amazonia Peruana IIAP. Perú. 49p. Disponible en: http://repositorio.iiap.org.pe/bitstream/IIAP/147/2/Rengifo_libro_2007.pdf [ Links ]
Rivera, L. 2012. Caracterización fitoquímica, farma céutica, y alimenticia de papa culebrera india (Dracontium spruceanum (Schott), G.H.Zhu, Araceae) y sande (Brosinium utile (Kunth) Oken, Moraceae) del Jardín Botánico de Plantas Medicinales del CEA de Corpoamazonia, Mocoa, Putumayo. Bogota, Colombia. 11 pp. [ Links ]
Rostagno, H.; Albino, L.; Donzele, J.; Gomes, P.; De Oliveira, R.; Lopez, D.; Ferreira, A.; Barreto, S.; Euclides, R. 2011.Tablas Brasileñas para aves y cerdos. Composición de alimentos y requerimientos nutricionales. 3a Edición. Universidad Federal de Viçosa–Departamento de Zootecnia. 259 pp. [ Links ]
Sakai, N.; Sakai, M.; Mohamad Haron, D.; Yoneda, M; Mohd, M. 2016. Beta agonists residues in cattle, chicken and swine livers at the wet market and the environmental impacts of wastewater from livestock farmers in Selangor state, Malaysia. Chemosphere 165: 183-190. [ Links ]
Sánchez-Moreno, C. 2002. Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Food Science and Tecnology International 8(3): 121-137. [ Links ]
Sandoval, C. 2012. Capacidad antioxidante del extracto atomizado de uña de gato (Uncaria tomentosa) y efecto sobre los perfiles bioquímicos sanguíneos, constants hematológicas y parámetros productivos en pollos de carne. Tesis. Pregado. Universidad Nacional Agraria de la Selva. Tingo María. Perú: 77 pp. [ Links ]
Skovgaard, N.; Hicks, J.; Wang, T. 2010. Oxygen uptake and transport in air breathers. In: Nilsson, G.E. Respiratory Physiology of Vertebrates. Cambridge University Press. CB. UK. p. 95-127. [ Links ]
Soltani, M.; Tabeidian, S.; Ghalamkari, G.; Adeljoo, A.; Mohammadrezai, M.; Saheb Fosoul, S. 2016. Effect of dietary extract and dried aereal parts of Rosmarinus officinalis on perfomance, immune response, and total serum antioxidant activity in broiler chicken. Asian Pacific Journal of tropical disease 6(3): 218-222. [ Links ]
Universidad Nacional de Córdova. 2016. Corporation Analytical Service: InfoStat [ Links ]
Vásquez, M.; Cueva, S.; Lira, B.; Ayón, M.; Rodríguez, J.; Angulo, P.; Falcón, N. 2012. Rol del óxido nítrico en la hipertrofia arteriolar pulmonar y ventricular cardiaca derecha en pollos a nivel el mar y expuestos a hipoxia de altura. Rev. Inv. Ve. Perú 23(1): 1-12. [ Links ]
Velandia, D. 2009. Evaluación cicatrizante y caracterización fitoquimica de Dracontium croatii, Tesis Postgrado. Universidad Nacional de Colombia. p 55-56. [ Links ]
Vielma, H.; Hernández, L.; Rodríguez, C.; Rivera, L. 2014. Propiedades inmunomoduladoras de la uña de gato. Planta 9(18):38–40. [ Links ]
Wagner, H.; Kreutzcam, P.; Juric, K. 1985. Alkaloides of Uncaria tomentosa and their phagocytosis increasing effect. Plant. Med. 51: 25-31. [ Links ]
Yunis-Aguinaga, F.; Claudiano, G.; Marcuso, P.F.; Ikefuti, C.; Ortega, G.; Eto, S.; de Cruz, C.; Moraes, L.; Moraes, F.; Fernades, J. 2014. Acute toxicity and determination of active constituent of aqueous extract of Uncaria tomentosa bark on Hyphessobrycon eques. Journal of toxicology ID 412437: 1-5. [ Links ]
* Corresponding author
E-mail: daniel.paredes@unas.edu.pe (D. Paredes-López).
Received August 10, 2017.
Accepted September 4, 2018.