Introduction
Viruses, unlike cellular bacteria, consist only of a single protein shell (called a capsid), which contains within its RNA segments that are instructed to invade a host cell. They are generally much smaller than bacteria, about a few nanometers in size (one nanometer (“nm”) is equivalent to 10-9 m) (Darnell and Taylor, 2006). Certain viruses are known to cause health problems for humans; and since they are not strictly cellular organisms, most antibiotics do not usually inactivate them. It is required in those cases to produce specific antibodies (vaccines) in order to be inactivated (Bodmer et al., 2018).
Viruses, when they do not have a host, can usually be present for a time on all kinds of surfaces; these include both biological and non-biological materials (plastics, metals, air, among others). This obviously also includes unprocessed foods, which often carry the virus on their surfaces (Hirneisen et al., 2011; Mullis et al., 2012a). For example, it has already been determined that viruses are often present in fruits and vegetables when they have been exposed to unsanitary environments. Presence of human viruses in treated sewage has been detected at concentrations of 104 to 105 virus particles/L (Hellmér et al., 2014; Wang et al., 2018; Wolf et al., 2019).
Many viruses inhabit animal species, which can be consumed and produce fatal diseases to the population. An example is a recent specific case of SARS-CoV-2 virus, also called Covid-19 (a virus belonging to the coronavirus family). There is reasonable evidence that it infected humans from the consumption of animal species such as bats (Li et al., 2020; Menachery et al., 2020; Yuan et al., 2020). Consumption of these species may have caused a mutation of the virus, possibly because they were consumed without proper treatment. This is not the only case where food consumption may have caused the spread of a virus in humans, e.g. viruses are known to exist in pigs (Leopardi et al., 2020; Zhou et al., 2019; Zhu et al., 2019), dromedary (Gossner et al., 2016), alpacas (Crossley et al., 2010) and avian products (Chumpolbanchorn et al., 2006), among others. In the case of the avian influenza virus (spherical virus with a size between 80 - 120 nm), it caused a zoonotic disease in Southeast Asia and throughout the world. In Southeast Asia, more than 60 people have died from the disease since the first case in Hong Kong was reported in 1997. In Thailand, there were 13 deaths in 21 cases reported from December 2003 to November 2005 (Kim, 2018; McDevitt et al., 2012).
Currently, there is a worldwide pandemic due to the Covid-19 coronavirus, which has caused a great impact on humanity in social, economic, and psychological aspects and unfortunately on health with an average mortality rate of 2.5% (Kitajima et al., 2020). The sources of contamination have generally been human-to-human contact (van Doremalen et al., 2018); but, there are other possible sources of contamination. For example, contaminate surfaces with the virus including biological materials such as food. No studies have been reported to date to efficiently disinfect Covid-19. Safe treatments to disinfect using inorganic or organic components should be investigated. The population is currently exposed to contamination from food that has been handled by virus-positive people, or from the use of contaminated water in cleaning (Mullis et al., 2012b). In fact, Covid-19 can be active for up to 9 days on surfaces that maintain its high infection rate and rapid spread (more rapid than SARS-CoV) (Kowalski et al., 2020).
In the disinfection of fruits and vegetables, inorganic chemical components such as hypochlorite and quaternary ammoniums are usually used; as well as organic stems such as, ethanol, propanol, among other components. Many of them have demonstrated their effectiveness in the elimination of viruses in food. With regard to the inactivation of Covid-19, there are no studies on the recommended doses of these disinfectants to inactivate it; however, effective doses have been reported for viruses of the same family of the coronavirus (MERS, SARS-CoV, and Murine Coronavirus). The similarity genomic structure of Covid-19 with respect to members of the same family is very appreciable (Henwood, 2020; Schmidt et al., 2005; Woo et al., 2010). For that, the disinfection doses tested for other members of its own family are expected to have a similar effect.
The indiscriminate use of chemical disinfectants can also have an impact on the environment and people. Waste is produced that must be treated before being disposed and it also can be toxic to human health. The World Health Organization (WHO) has recommended caution in the use of disinfection methods because the population may informally use indiscriminate concentrations of disinfectants that are not suitable for eliminating coronaviruses (including Covid-19); they may lead to an increase in cases of disinfectant poisoning.
This review presents a summary of the scientific evidence on the treatment of food with inorganic or organic disinfectants, which have been used to specifically inactivate viruses of the coronavirus family (MERS, SAR-CoV, Murino coronavirus); as well as other viruses, such as HnNn influenza, swine influenza or avian influenza. Although, there are no specific studies for the Covid-19 virus in the international scientific community, this review may be useful to the industry in the search for disinfectants and their most recommended doses to treat viruses in the Covid-19 family - the coronaviruses (COVs). The present work aims to show the use of different types of disinfectants and their dosages, in the food industry; as well as, to discuss the advantages and disadvantages of each disinfectant with respect to environmental sustainability.
Coronaviruses classification
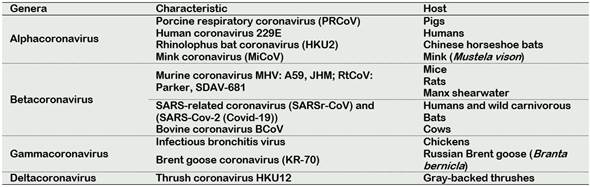
The ability of viruses to infect the human host is an important reason that the WHO makes top list of emerging diseases likely to cause major epidemics. The list includes severe acute respiratory syndrome coronavirus (SARS-CoV), and recently Covid-19 which caused the current pandemic (Gravemann et al., 2018; Mullis et al., 2012b). SARS-CoV viruses belong to the Orthocoronavirinae species, Coronaviridae family, and Nidovirales order; they are characterized by being large, and enveloped single-stranded RNA. Based on genotypic and serologic characterization, CoVs can be subdivided into the genera Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus (Crossley et al., 2010). CoVs are defined as enveloped viruses of 120 -160 nm in diameter with a crown-like appearance. The name “coronavirus” is derived from the Greek “κορώνα”, meaning crown and containing the largest genomes (26.4 to 31.7 kb) among all known RNA viruses, with G + C contents varying from 32% to 43% (Henwood, 2020). The differences between genera are by the genome organization, Alpha-, Beta-, Gamma and Delta prototypes (Decaro, 2011; Poletti and Mavilio, 2017). In Table 1, common viruses for genera Alphacoronavirus, and their respective host, are listed.
Due to the current pandemic 2020, the most important virus to be monitored by the food industry is the CoVs, mainly the SARS-CoV and Covid-19. They are very contagious, and they are deadly to humans. SARS-CoV and Covid-19 are classified in the genera Betacoronavirus, due to that they show a beta prototype in their genome structure.
Risk of Coronavirus and influenza virus in food
Pre-harvest contamination for foodborne viruses can occur through a variety of agents, including animal feces, manures, soil, irrigation water, animals, and human handling. Problems of contamination are magnified by potential countrywide distribution. Postharvest processing of produce can involve spraying, washing, or immersion into water with disinfectants; however, disinfectants, including chlorine, have varying effects on viruses and produce harmful by-products pose a concern (Hirneisen et al., 2011).
Transmission of CoVs in humans is generally airborne or by person-to-person contact through droplets of saliva, sneezes, coughs, and secretions that can contaminate hands and surfaces. However, currently the food industry is facing numerous uncertainties about of the presence of Covid-19 in food production, distribution, marketing, home preparation of meals (Oliveira et al., 2020) or in the generation of wastewater (Kitajima et al., 2020). Coronavirus infections have a significant economic impact on agriculture, human health, and social impact in the word. In the early 1980’s the bovine coronavirus (BCoV) began to heavily impact food agriculture, due to the apparition of viral gastroenteritis in cattle (Clark et al., 1998). In fact, BCoV had a major impact on the cattle industry, with economic losses occurring due to morbidity, mortality, treatment and prevention costs, loss of production, and reduced carcass value (Boileau and Kapil, 2010; Fulton et al., 2011). Therefore, presence of BCoV in the bovine or calves can be a source of virus contamination in humans when they are consuming beef as a food. As well as fruits (Brie et al., 2018) and vegetables (Mullis et al., 2012b) can be a food source of CoVs contamination. The need to adopt measures to reduce the risk of a breakdown in food supply services is required. Recently WHO reported that the Hepatitis E virus, Highly Avian influenza, H5N1 influenza virus, SARS-CoV and Nipah virus, as pathogenic having the potential for foodborne transmission. Initial foodborne transmission is a route to enter the human population, which can then shift and spread through human-to-human transmission.
The generation of studies for prevention of CoVs infections due to food contamination requires adherence to the countries in the world and by programmers who consume. Because a vaccine to inactivate CoVs in humans is not yet available, the food sanitization programs in the entire world should be reformulated to decrease the risk of infections by the presence of CoVs in food. Fast protocols to prevent the possibility of contamination of viruses (i.e. by coronaviruses) from food are to be researched.
Inactivation of CoVs and influenza virus, using common disinfectants
Fruits and vegetables are heavily consumed and have good nutritional value. The perishable nature of these products, which determines its fast consumption and also the number of associated outbreaks, identifies the importance of applying efficient decontamination strategies (Mullis et al., 2012b).They can be treatments with heat to destroy the viruses. Another alternative has been the use of chemical disinfectants, in the case of fresh food.
Use of free chlorine and derivate
Free chlorine readily penetrates the lipid membrane of microorganism to react with proteins in the nucleocapsid and polymerase complex (Ye and Wigginton, 2018). In the case of a virus, free chlorine damaged viral capsids, allowing free chlorine access to viral RNA to damage viral genomes and cause it to lose its ability to bind to its host receptors (Fuzawa et al., 2019). Chlorine can be used in a gas form (chlorine dioxide) or liquid form (hypochlorite). Chlorine dioxide is regarded as a broad-spectrum disinfectant with strong inhibitory effects on microbes and parasites, and is an efficient agent and potently suppressed porcine reproductive and respiratory syndrome virus infection in vitro (Kingsley et al., 2018; Zhu et al., 2019). Chlorine dioxide (ClO2) is commonly applied to food or irrigation water which has the advantage of not forming organo-halogen by-products, and it is a more powerful oxidant than sodium hypochlorite (Lopez-Galvez et al., 2018).
Liquid Sodium hypochlorite (NaOCl) is another chlorine compound used as a disinfectant. It has an effective antimicrobial activity, a broad bacterial range, and creates a significant reduction in endotoxins levels (Neelakantan et al., 2019). However, several studies have demonstrated that complete bacterial elimination cannot be achieved consistently with any of the current disinfection protocols (Silva et al., 2020; Siqueira et al., 2018). It is important to note that chlorination of water that contains amino acids have been shown to produce toxic cyanogen chloride. Dissolved organic nitrogen substances can also react with chlorine to give organo-chloramines. Peptides in water are of greater concern than free amino acids because chlorinated products of peptides are relatively more stable than the chlorinated derivate of amino acids (Sharma and Graham, 2010).
Use of Alcohol
Most alcohols used in disinfection are ethanol and isopropyl alcohol, both usually at a concentration of 70%; for example, the transmissible gastroenteritis virus (TGEV) was reduced 4.5 log10 by ethanol in the suspension test, over 5 minutes. Similarly, using QCT-2 and organic load, ethanol was reduced by at least 3 log10 over the course of 5 minutes (Schmidt et al., 2005). Ethanol is widely used in hand rubs, gels, and foams for hand hygiene in healthcare settings. In fact, The WHO has even listed ethanol at 80% (v/v) as an essential medicine in the category ‘alcohol-based hand rubs’ and since 1994, the US Food and Drug Administration considers ethanol between 60% and 95% as generally safe and effective (Kampf, 2018). It has been noted that coronaviruses, such as 0.05% to 0.2% benzalkonium chloride or 0.02% chlorhexidine di-gluconate were less effective. Coronaviruses, e.g. severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), can be inactivated by 70% ethanol and 0.1% sodium hypochlorite; for that, it is believed that Covid-19 would be similarly affected (Kampf et al., 2020).
Use of quaternary ammonium
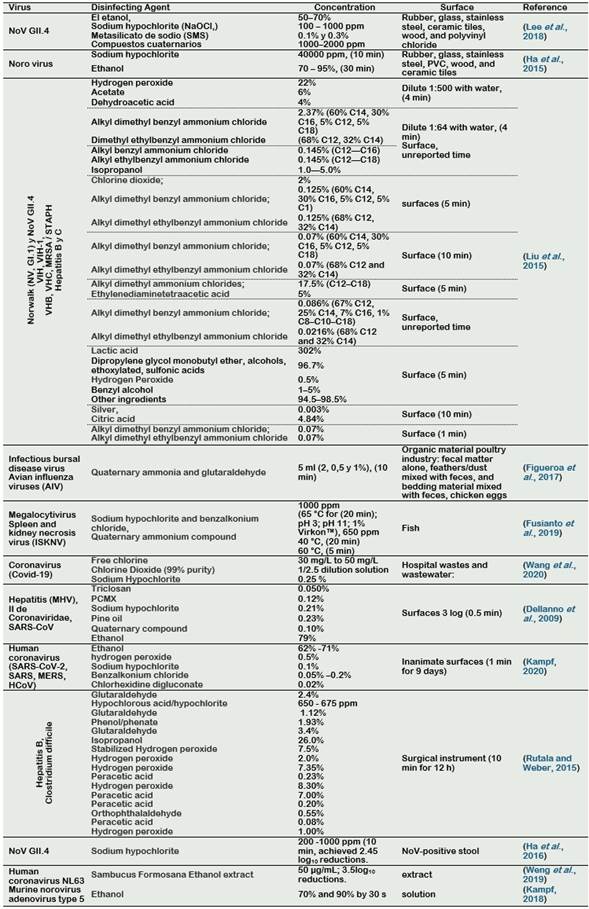
Quaternary ammonium cations are another type of disinfectant that have spermicidal and antimicrobial activity and inactivation of encapsulated viruses, especially those containing long alkyl chains (Mizzen et al., 1985). Examples are benzalkonium chloride, benzethonium chloride, methylbenzethonium chloride, cetalconium chloride, cetylpyridinium chloride, cetrimonium, cetrimide, tetraethylammonium bromide, didecyldimethylammonium chloride and domiphene bromide. Alam et al. (2018) showed that the quaternary ammonium (diluted at 1:500 (QACx500)) inactivated the avian influenza on steel and plastic carriers after 60 minutes of exposition. In Table 2, the applications of common disinfectants to inactivate common coronaviruses and influenza viruses are shown.
Use of Ozone (03)
Ozone is a powerful antimicrobial agent that is suitable for application in food in the gaseous and aqueous states. It is one of the most effective sanitizers known, yet it leaves no hazardous residues on food or food-contact surfaces. The precursors for industrial production of ozone (that is, O2 or HO) are abundant and inexhaustible. Ozone treatment requires no heat and hence saves energy (Hirneisen et al., 2011; Khadre et al., 2001; Wang et al., 2018). Gaseous ozone has a residual effect, for example its half-life based on temperature in Gaseous is at 8 days at -25 °C, 3 days at 20°C and 1.5 hours at 120 °C. In aqueous ozone it is at 30 minutes at 15 °C, 20 minutes at 20 °C and 15 minutes at 25 °C (Miller et al., 2013). Aqueous ozone has been used to decontaminate poultry meat (Ianni et al., 2019), salmon (Crowe et al., 2012), apples (Camargo et al., 2019), strawberries (Nayak et al., 2020) , and others foods (Mahapatra et al., 2005). Hirneisen et al. (2011) tested ozone for the processing of lettuce and reported that bubbling ozone reduced counts of natural microflora in the range of 2 to 3 log10. Also, it has been used to prevent mold growth on cheese and inactivate airborne molds in cheese ripening and storage facilities. Ozone treatment has also been found to be a promising method for reducing the concentrations of pollutants in dairy wastewaters (Varga and Szigeti, 2016). In Table 3, treatments to inactivate coronaviruses and influenza virus, by 03, is showed.
Ozone is a strong oxidative compound both in its gaseous form and when dissolved in water and, because of this, is known to be an efficient disinfectant for inactivating even the chemically resistant. Its oxidative potential is higher than that of hydrogen peroxide and hypochlorite (Mahfoudh et al., 2010). However, concentration levels of 03 and residence times must be controlled, because it is toxic in humans at high doses. One-hour exposure to ozone concentrations of 2, 4, 15, and 95 ppm induces symptomatic, irritant, toxic, and irreversible lethal effects in humans, respectively. In fact, the human lung is the primary target of ozone gas (Khadre et al., 2001). The Food and Drug Administration (FDA) requires a concentration limit exposure of 0.05 ppm during 8 hours, Occupational Safety and Health Administration (OSHA) requires a concentration limit exposure of 0.10 ppm during 8 hours, National Institute of Occupational Safety and Health (NIOSH) recommends an upper limit of 0.10 ppm, not to be exceeded at any time, Environmental Protection Agency (EPA) requires a concentration limit exposure of 0.08 ppm during 8 hours (Miller et al., 2013).
Use of ultraviolet light (“UV”) as virus disinfectant
Ultraviolet light can be an effective disinfectant for decontaminating virus surfaces, including the SARS-CoV virus. It is possibly because UV inducing photo dimers in the genomes of microorganisms structure (Darnell et al., 2004). Ultraviolet light has been demonstrated to be capable of destroying viruses, bacteria and fungi in hundreds of laboratory studies (McDevitt et al., 2012). Although SARS-CoV-2 virus has not yet been specifically tested for its ultraviolet susceptibility, other tests on related coronaviruses, including the SARS coronavirus, have concluded that they are highly susceptible to ultraviolet inactivation (Kowalski et al., 2020). UV light is divided into three classifications: UV-A (320 - 400 nm), UV-B (280 - 320 nm), and UV-C (200 - 280 nm). UV-A is weakly absorbed by DNA and RNA, and is much less effective than UV-C and UV-B. Duan et al. (2003) found that irradiation with ultraviolet light for 60 minutes on several coronaviruses in culture medium resulted in undetectable levels of viral infectivity.
UV is uniquely vulnerable to the virus at wavelengths near to 253.7 nm (UV-C range). This is because the maximum absorption wavelength of a DNA molecule is around to 260 nm. UV-A and UV-B require more exposure time to be effective against viruses. Also, airborne microorganisms are much more susceptible to UV damage than those suspended in a liquid suspension; additionally, the type of the microorganism, affected the efficacy of UV-C. For example, the UV-C dose between fungal spores and bacterial cells is as high as 80 times (Tseng and Li, 2005).
Similarly, to the other disinfectants, human exposure to high levels of UV-C irradiation is dangerous to their health. Overexposure to ultraviolet (UV) radiation is the main modifiable risk factor for skin cancer (Lehmann et al., 2019). Exposure to UV-C is not recommended for humans at levels of 2.5 J/m2 by 10 to 15 minutes can produce erythema (redness of the skin or mucous membranes) (McKenzie and Lucas, 2018). The UV-A dosage was set to levels calculated safe for human occupation at maximum of 10 W m2 for 8 hours, at eye level (Brons et al., 2020). In Table 4, some treatment to inactivate coronaviruses and influenza virus by UV are showed.
Table 3 Common virus and its corresponding treatment with ozone
| Viruses | Ozone (ppm or mg/L) | Time (min) | Medium | Log10 reduction | Reference |
|---|---|---|---|---|---|
| Murine Coronavirus | 10 | 10 | Air (90 %) | > 3 | (Hudson et al., 2009) |
| Murine norovirus MNV-1 | 6.25 | 5 | green onion | 1.61 | (Hirneisen et al., 2011) |
| Coronavirus (Covid-19) | 15 - 20 | 10 - 15 | air | 3 | (Wang et al., 2020) |
| Murine norovirus | 6.25 | 5 | lettuce | 2.91 | (Hirneisen et al., 2011) |
| Murine norovirus | 6.25 | 5 | water | 4.69 | (Hirneisen et al., 2011) |
| Respiratory syncytial virus | 0.5 | 60 | air | (Becker et al., 1998) | |
| HSV | 10 | 15 | plastic | 4 | (Hudson et al., 2009) |
| HSV | 10 | 15 | glass | 4 | (Hudson et al., 2009) |
| HSV | 10 | 15 | stainless steel | 4 | (Hudson et al., 2009) |
| Influenza virus | 10 | 15 | plastic | 4 | (Hudson et al., 2009) |
| Influenza virus | 10 | 10 | glass | 4 | (Hudson et al., 2009) |
| Influenza virus | 10 | 20 | stainless steel | 4 | (Hudson et al., 2009) |
| Rotavirus | 10 | 10 | plastic | 4 | (Hudson et al., 2009) |
| Rotavirus | 10 | 15 | glass | 4 | (Hudson et al., 2009) |
| Rotavirus | 10 | 20 | stainless steel | 4 | (Hudson et al., 2009) |
| Bacteriophage MS2 | 0.6 | 0.3 | water | 2.96 | (Finch and Fairbairn, 1991) |
| Hepatitis A virus | 0.3 to 0.4 | 0.08 | water | 3.9 | (Hall and Sobsey, 1993) |
| Poliovirus type 3 | 0.3 | 0.3 | water | 1.63 | (Finch and Fairbairn, 1991) |
| Bacteriophage MS2 | 1.2 | 60 | air (85% h) | 3 | (Mamane et al., 2007) |
| Bacteriophage PHIX174 | 1.2 | 30 | air (85% h) | 3 | (Mamane et al., 2007) |
| Bacteriophage PHI6 | 1.2 | 30 | air (85% h) | 3 | (Mamane et al., 2007) |
| Bacteriophage T7 | 1.2 | 120 | air (85% h) | 3 | (Mamane et al., 2007) |
| Murine norovirus | 3 | 1 | Raspberries | > 3.3 | (Brie et al., 2018) |
| hepatitis A (HAV) | 5 | 3 | Raspberries | 0.6 | (Brie et al., 2018) |
| Adenovirus, | 6 | 30 | wastewater | 5 | (Wang et al., 2018) |
| Norovirus, | 7 | 15 | wastewater | 5 | (Wang et al., 2018) |
| Parechovirus | 6 | 15 | wastewater | 5 | (Wang et al., 2018) |
| Sapovirus | 6 | 15 | wastewater | 5 | (Wang et al., 2018) |
Table 4 Common virus and it corresponding treatment with UV
| Viruses | UV dose | Time (min) | Medium | Log10 reduction | Reference |
|---|---|---|---|---|---|
| SARS CoV Urbanus | 241 J/m2 | 1 | air | 3 | (Kowalski et al., 2020) |
| Coronavirus | 7 J/m2 | 1 | air | 3 | (Walker and Ko, 2007) |
| SARS CoV | 40.16 J/m2 | 6 | air | 3 | (Darnell et al., 2004) |
| Canine coronavirus | 29 J/m2 | 1 | air | 3 | (Kowalski et al., 2020) |
| SARS CoV | 1.7 J/s m2 | 20 | plasma | > 3.4 | (Eickmann et al., 2020) |
| Murine Coronavirus | 29 J/m2 | 1 | air | 3 | (Kowalski et al., 2020) |
| SARS CoV Hanoi | 134 J/ m2 | 1 | air | 3 | (Kowalski et al., 2020) |
| Nipah virus | 1.7 J/s m2 | 20 | plasma | > 4.3 | (Eickmann et al., 2020) |
| Haemorrhagic fever virus | 1.7 J/s /m2 | 20 | plasma | > 2.2 | (Eickmann et al., 2020) |
| Berne Virus | 7 J/m2 | 1 | air | 3 | (Kowalski et al., 2020) |
| T7 virus | 20 J/s m2 | 0.017 | air 55% RH | 3 | (Tseng and Li, 2005) |
| Phi6 virus | 17.7 J/s m2 | 0.017 | air 55% RH | 3 | (Tseng and Li, 2005) |
| SARS CoVP9 | 1 J /s m2 | 30 | (Duan et al., 2003) | ||
| MS2 virus | 9 J /s m2 | 0.0107 | air 55% RH | 3 | (Tseng and Li, 2005) |
| PhiX174 virus | 10.3 J/s m2 | 0.017 | air 55% RH | 3 | (Tseng and Li, 2005) |
| Avian influenza virus aiv H1N1 | 15 J/m2 | 15 | air | 2 | (McDevitt et al., 2012) |
| Avian influenza virus aiv H7N1 | 0.9 J/m2 UVB | 158 | air | 1 | (Sutton et al., 2013) |
| Avian influenza virus aiv H5N1 | 0.9 J/m2 UVB | 167 | air | 1 | (Sutton et al., 2013) |
| Influenza viruses | 23 J/m2 | 20 | air | 3 | (Sutton et al., 2013) |
From the Tables 2 to 4, we can observe that doses to inactive CoVs can vary depending on the sample conditions and the raw materials used in the experiments. According to Table 2, CoVs could be inactivated using free chlorine solutions at 30 mg/L, sodium hypochlorite 0.25 %, or Chlorine Dioxide (99% purity) diluted at 1/2.5 relation (Wang et al., 2020). Also, alcohol is an effective disinfectant of CoVs, it can inactivate at concentrations of 62 to 71 % of ethanol (Kampf, 2018; Kampf et al., 2020). With respect to the use of the Quaternary compound, it can be used at concentrations of 0.10% (Dellanno et al., 2009). In addition, the use of the Sambucus Formosana ethanol extracts, at 50 µg/mL, can be used to inactivated CoVs (Weng et al., 2019).
The use of Ozone is another promising disinfectant to inactivate CoVs and Covid-19. Doses of 03 between 10 to 20 ppm for times of 10 to 15 minutes are recommended to inactivate CoVs at 3.5 log10 reductions. However, a warning should be reported for use of high doses and exposure because it can be a risk to human health (limited exposure to humans is 0.05 ppm for 8 hours).
From Table 4, ranges of UV-C dose, for inactive CoVs; is around 7-241 J/m2, with a mean of 67 J/m2. These values can be taken as an adequate representation of the ultraviolet susceptibility of the SARS-CoV- 2 (Covid-19) virus (Kowalski et al., 2020). With respect to the time exposure, UV-C shows ranges between 1 to 30 minutes of exposure. In early April, the SSLEEC member company Seoul Semiconductor, reported that a 99.9% of the Covid-19 sterilization can be reached at 30 seconds, using UV LED products.
Conclusions
Currently, there is a worldwide pandemic due to the Covid-19 coronavirus, which has caused a great impact on humanity in social, economic, psychological aspects and unfortunately on health. Due to the risk that food can also be a medium to cause a virus disease, the procedures in the food industry safety programs must be revised; and be more specific on how to disinfect for Covid-19. Some effective disinfectants that have been proved to inactivate CoVs are chlorine dioxide, sodium hypochlorite, quaternary compound, ozone, and UV-C. Further research is still needed to explore new applications for these disinfectants and to best utilize the unique features of these sanitizers. For example, using the disinfectants in binary and/or ternary mixtures, to evaluate their synergies. Similarly, the efficiency of these mixtures in the inactivation of Covid-19 should be explored.