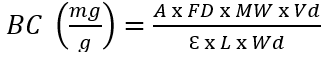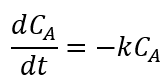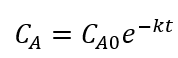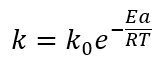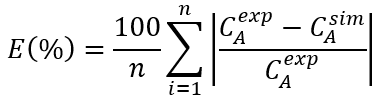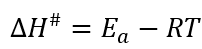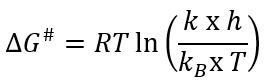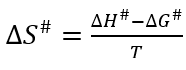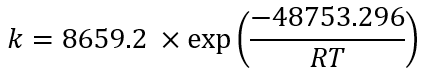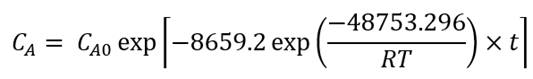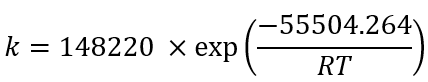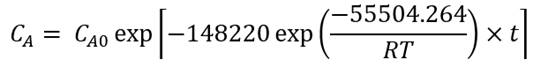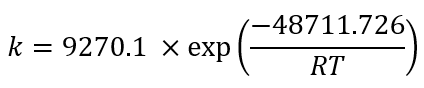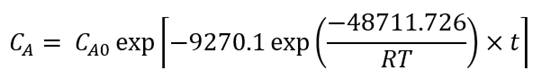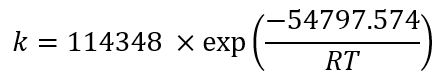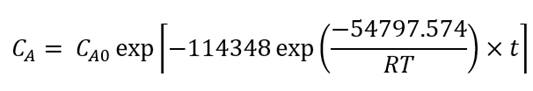1. Introduction
Betalains, carotenoids and anthocyanins are the main natural pigments used in the food industry that have shown biological effects, especially in the prevention and treatment of chronic diseases such as diabetes, obesity and cardiovascular diseases (De Mejia et al., 2020). Current trends in the development of natural food additives imply the evaluation of phenolic compounds that contribute to antioxidant activity and enhance their functional food market value (Caldas-Cueva et al., 2016). Beetroot (Beta vulgaris L.) has antioxidant properties, as it contains water-soluble nitrogen pigments called betalains. These are classified into two main groups, the red betacyanins and the yellow betaxanthins; these are considered scarce in nature, because they can only be found in beetroot (Beta vulgaris L.), the seeds and leaves of amaranth (Amaranthus sp.) and in some cactus of the genera Opuntia and Hylocereus, such as the purple cactus pear and the pitaya (Cai et al., 1998; Stintzing et al., 2002). Recent studies have shown evidence that the intake of beetroot offers beneficial physiological effects that improve the clinical results for different pathologies such as hypertension, atherosclerosis, type II diabetes and dementia (Clifford et al., 2015). According to German regulation about additives, the use of betalains as pigments is only allowed in some foods and in defined amounts; it is known as the natural food colouring number E162 (Esatbeyoglu et al., 2015). Pigment stability is an important aspect to be considered when using these pigments because the intensity and antioxidant activity are affected by factors such as pH, water activity, enzymes, oxygen and temperature during processing and storage; also, they tend to be easily degraded in solution (Azeredo, 2009). The application of natural red colorants in foods is broad; however, their stability depends on the food matrix and storage conditions (Von-Elbe et al., 1974). In order to protect these bioactive compounds against undesirable reactions and to extend their shelf life, the microencapsulation process by spray drying has been used (Escalona-García et al., 2016; Esquivel-González et al., 2015). It has been said that the stability of betalains can be improved by using the aforementioned process (Desai & Park, 2005). The thermal treatment is the most common and efficient way to preserve foods; however, it can result in a loss of quality, mainly related to the rapid degradation of bioactive compounds (Patras et al., 2010; Sadilova et al., 2007). This is also the most important factor affecting the stability of betalains and other phenolic compounds (Azeredo, 2009). As a result, it is necessary to preserve the content of betalains during thermal processing, which should be strong enough to destroy the microorganisms and enzymes of interest yet gentle enough to avoid chemical changes that damage the flavour and nutritional value during long storage periods (Costa et al., 2018). One of the most useful tools for the kinetic degradation of pigments and vitamins during the storage period, is the use of the kinetic principles of chemical reactions (Labuza & Riboh, 1982; Ramaswamy et al., 1989; Van-Boekel, 1996). Kinetic models have been studied on the degradation of betalains from the peel of pitaya and betalains from beet extract applied as a colorant in milk, the results demon strated the following of a first-order reaction kinetics (Chew et al., 2019; Güneşer, 2016) but there are few studies that contribute to the kinetic and thermodynamic analysis of betalains degradation in microencapsulated beetroot juice. In this study, the thermal degradation of betalains as well as the kinetic and thermodynamic parameters in microencapsulated beetroot juice were determined under a low range of temperatures, the above because there is a wide range of possible application under the studied temperatures. The use of the microencapsulation process offers the advantage of being an efficient and protective method for sensitive compounds like betalains and its potential use as a food additive. The objective of the present study was to provide a mathematical model that can simulate the degradation of betalains, which could be a useful tool for analysing the operative conditions and to consequently reduce the losses of betalains in microencapsulated beetroot juice.
2. Materials and methods
2.1 Sample preparation and heat treatment
Beetroot (Beta vulgaris L.) was acquired from a local market in the city of Durango, Dgo. It was washed with potable water, peeled and reduced in size, and then processed by an extractor (Turmix, rough use model u4087294, Mexico) to obtain the juice. To 40 mL of beetroot juice, maltodextrin 10 DE solution (10% w/v) and sweet potato starch solution (2% w/v) were added in proportions of 40MDX:60SPS, 20MDX:80SPS and 0MDX:100SPS, resulting in a final volume of 100 mL for each mixture, which was fed to the spray dryer. The mixtures were microencapsulated by a spray dryer (Büchi Mini Spray Dryer Model B-290, Switzerland) with an inlet air temperature of 150 °C and a feed flow rate of 8 mL/min. Another portion of the juice was lyophilized (Freeze dryer, model 117 A65312906, USA) with maltodextrin 10 DE solution (4% w/v) and sweet potato starch solution (1.2% w/v) to be used as a control. For the extraction of betalains from the powders, the method of Esquivel-González et al. (2017) was followed with some modifications. Briefly, 100 mg of powder was placed in screw cap tubes with a diameter of 2 cm containing 20 mL McIlvaine buffer adjusted to a pH of 3.5 and centrifuged at 3500 rpm for 15 min (Corning Model LSE, Germany). Finally, the supernatant was recovered. The supernatant was used to carried out thermal degradation at temperatures of 6, 19 and 30 °C; this was performed in duplicate. The process was evaluated for one and a half hours, and the samples for analysis were taken at 0, 15, 30, 45, 60, 75 and 90 min for each temperature analysed.
2.2 Quantification of betalains
The betalains content of the samples was measured by the method of Castellanos-Santiago & Yahia (2008) with some modifications; analysis involved the use of a spectrophotometer (Hach, model DR 5000, USA). The absorption spectra were recorded at 535 nm for betacyanins and 483 nm for betaxanthins until absorption values ≤ 1.1 were obtained. The measurements were made in duplicate and the total content of betalains (mg/g) was calculated using equation 1.
Where A is the absorbance value, FD is the dilution factor, MW is the molecular weight (550 g/mol for betacyanins and 380 g/mol for betaxanthins), Vd is the volume of the solution (mL), Ɛ is the molar extinction coefficient (60,000 L/mol/cm for betacyanins and 48,000 L/mol/cm for betaxanthins), L is the length of the cell (cm) and Wd is the weight of the sample (g). To obtain the total betalains content, the results of betacyanins and betaxanthins are added.
2.3 Kinetic modelling
The kinetic model considered follows the first order model and was based on the classic approach used in chemical reaction engineering expressed in equation 2, which can be easily integrated to equation 3. This model was chosen based in literature works dealing with the degradation kinetics of biocompounds for example betalains from beet extract (Chew et al., 2019).
Where C A expresses the total betalains concentration (mg/g) at any time t, C A0 is the initial concentration of betalains and k is the degradation rate constant that depends on temperature according to Arrhenius law (Eq. 4).
Where k 0 is the pre-exponential factor (h), E a is the activation energy (J/mol) and R is the gas constant (8.314 J/mol/K). The model parameters were identified using nonlinear regression on the logarithmic curves of experimental data. To determine the reproducibility of the models, the average errors between the experimental values and the values predicted by the models were calculated by equation 5. In addition, the determination coefficients (R 2 ) were also calculated for each model.
Where E is the average error, n is the number of experimental data, C A exp are the experimental values of total betalains concentration and C A sim are the simulated values using the models.
2.4 Thermodynamic analysis
The activation enthalpy (ΔH#) and the free energy of inactivation (ΔG#) at each temperature analysed were obtained using equations 6and7.
Where h (6.6262x10-34 J/s) is the Planck’s constant and k B (1.3806x10-23 J/K) is the Boltzmann’s constant. Then, the activation entropy (ΔS#) was calculated using equation 8.
3. Results and discussion
3.1 Kinetic analysis
The kinetic parameters were determined using the Excel exponential adjustment for the first-order model considered in this investigation. Figure 1 shows the experimental data for each analysed temperature and the exponential adjustment of each proportion of microencapsulating material used. In the same way, an exponential evolution of C A for the experimental data is shown, which suggests that this model can be used to represent the process by simulation. The higher content of betalains was retained when the proportion of 20MDX:80SPS was used as microencapsulating material. In all cases, the content of betalains decreased as the temperature and time of exposure to heating increased, indicating an effect of temperature and the availability of oxygen on the degradation of the red colour (Buvé et al., 2018). This finding agrees with those reported in previous studies with other types of fruits; Chew et al. (2019) studied betalains in beet extract, Summen & Erge (2012) studied anthocyanins in raspberry pulp, Silva et al. (2016) studied anthocyanins in acerola pulp, Vieira et al. (2015) worked with ascorbic acid in orange juice, and Azeredo (2009) mentioned that beetroot and red pitaya betalains showed a degradation behaviour in first-order reactions.
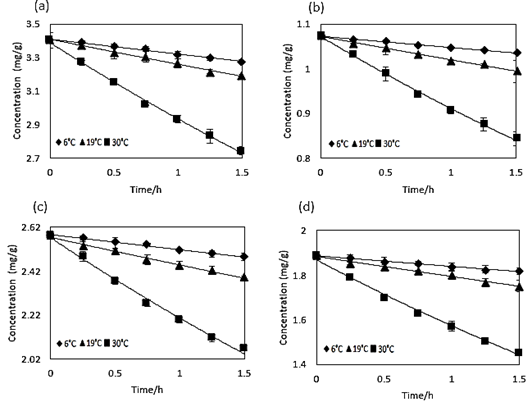
Figure 1 First-order kinetics of betalains thermal degradation in a) Beetroot juice, b) 40MDX:60SPS, c) 20MDX:80SPS, and d) 0MDX:100SPS at different temperatures. Experimental points of betalains concentration (mg/g) as a function of time expressed in hours and the adjusted exponential models as continuous lines.
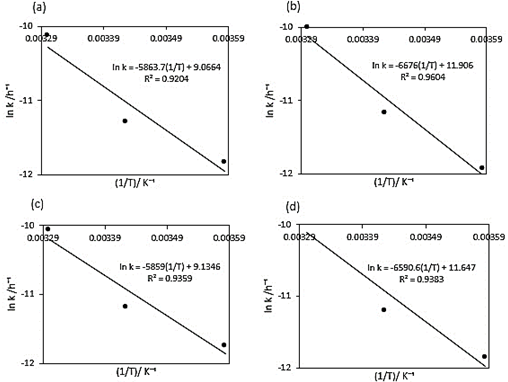
Figure 2 Arrhenius plot for temperature dependence of the degradation rate constant k: a) Beetroot juice, b) 40MDX:60SPS, c) 20MDX:80SPS, and d) 0MDX:100SPS.
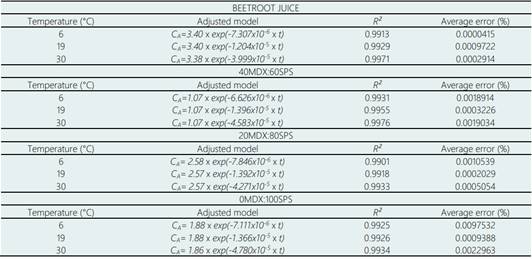
For all the curves in Figure 1, the coefficients of determination (R 2 ) were higher than 0.99 (Table 1). Also, Table 1 shows the average error in the reproduction of experimental data for the adjusted models. All the average errors were lower than 1%, which confirms that first-order kinetics is a good way to reproduce the degradation of betalains in microencapsulated beetroot juice. Table 1 shows the adjusted kinetic models from the experiments; each presents the pre-exponential factor (C A0 ). In the case of beetroot juice, the value for C A0 was 3.40 mg/g for all the temperatures tested, for the microencapsulated powders with 40MDX:60SPS it was 1.07 mg/g, for 20MDX:80SPS it was 2.57 mg/g, and for 0MDX:100SPS the value was 1.88 mg/g. Additionally, the kinetic rate constant (k) increases as the temperature also increases, in all experiments.
The initial content of betalains in the beetroot juice at 6 °C decreased by 3.61% with a kinetic rate constant of -7.307x10-6 1/h, at 19 °C decreased by 6.55 % with a kinetic rate constant of -1.204x10-5 1/h, and at 30 °C decreased by 20.35% with a kinetic rate constant of -3.999x10-5 1/h. The initial content of betalains in the powders with 40MDX:60SPS at 6 °C decreased by 3.64% with a kinetic rate constant of -6.626x10-6 1/h, at 19 °C decreased by 7.82 % with a kinetic rate constant of -1.396x10-5 1/h, and at 30 °C decreased by 21.8% with a kinetic rate constant of -4.853x10-5 1/h. The initial content of betalains in the powders with 20MDX:80SPS at 6 °C decreased by 3.68% with a kinetic rate constant of -7.846x10-6 1/h, at 19 °C decreased by 7.51% with a kinetic rate constant of -1.396x10-5 1/h, and at 30 °C decreased by 20.28% with a kinetic rate constant of -4.853x10-5 1/h.
The initial content of betalains in the powders with 20MDX:80SPS at 6 °C decreased by 3.68 % with a kinetic rate constant of -7.846x10-6 1/h, at 19 °C decreased by 7.51 % with a kinetic rate constant of -1.396x10-5 1/h, and at 30 °C decreased by 20.28% with a kinetic rate constant of -4.853x10-5 1/h. Finally, the initial content of betalains in the powders with 0MDX:100SPS at 6 °C decreased by 3.86 % with kinetic rate constant of -7.111x10-6 1/h, at 19 °C decreased by 7.31% with a kinetic rate constant of -1.366x10-5 1/h, and at 30 °C decreased by 23.2% with a kinetic rate constant of -4.780x10-5 1/h.
Figure 2 provides the kinetic rate constant as a function of the inverse of temperature to identify the Arrhenius parameters (k 0 ) and the activation energy (E a ).
Using the exponential adjustment of EXCEL, it is possible to obtain the Arrhenius parameters. During heating from 6 °C to 30 °C for beetroot juice, values of k 0 = 8659.2 h and E a = 48.75 kJ/mol were obtained, with a determination coefficient (R 2 ) equal to 0.9204. For powders with 40MXD:60SPS had values of k 0 = 148220 h and E a = 55.50 kJ/mol, with a determination coefficient (R 2 ) equal to 0.9604. For powders with 20MXD:80SPS, there were values of k 0 = 9279.10 h and E a = 48.71 kJ/mol, with a determination coefficient (R 2 ) equal to 0.9359. For powders with 0MXD:100SPS, there were values of k 0 = 114348 h and E a = 54.79 kJ/mol, with a determination coefficient (R 2 ) equal to 0.9383. The value of E a is a measure of the sensitivity of the reaction to temperature; it means, the lower the activation energy, the lower the sensitivity to degradation. In this study, it was found that the lowest value of E a corresponds to the kinetic degradation of the powder with 20MDX:80SPS.
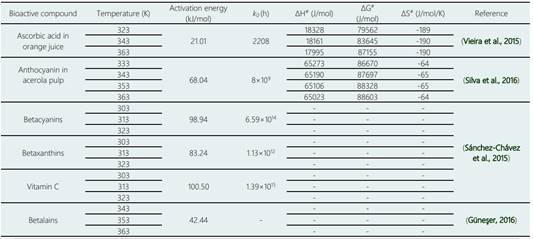
Sánchez-Chávez et al. (2015) reported an activation energy of 98.94 kJ/mol for betacyanins degradation and 83.24 kJ/mol for betaxanthins in a beetroot beverage during heating from 30 to 50 °C and Güneşer (2016) obtained an activation energy value of 42.44 kJ/mol for betalains in milk during heating from 70 to 90 °C (see Table 3), these values showed variability compared to those obtained in this investigation and it is attributed to that the used temperatures in these experiments were lower.
The Arrhenius equation for beetroot juice and powders with 40MDX:60SPS, 20MDX:80SPS and 0MDX:100SPS are shown in equations 9,11,13and15, respectively, and equations10,12,14and16 calculate the betalains concentration as a function of time and temperature during batch heating processes. Some factors that contribute to the wide distribution of the kinetic parameters are the intrinsic characteristics of the raw material, such as the variety, maturity, pH and probably the dissolved oxygen levels (Burdurlu et al., 2006; Dhuique-Mayer et al., 2007).
3.2 Thermodynamic analysis
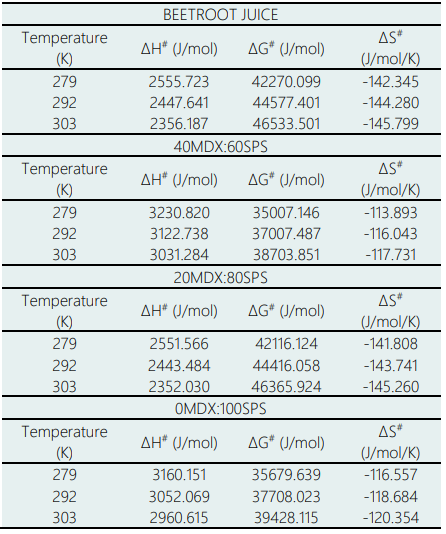
To verify that the kinetic model used in this research is thermodynamically possible, thermodynamic parameters were estimated on the kinetics of the thermal degradation of betalains in beetroot juice and in powders with different proportions of microencapsulating materials. Table 2 presents the activation enthalpy (ΔH#), the free energy of activation (ΔG#) and the activation entropy (ΔS#) at 6, 19 and 30 °C.
The free energy of activation (ΔG#), which represents the difference between the activated state and the reactants (Al-Zubaidy & Khalil, 2007), the beetroot juice showed values ranging from 42270.09 and 4653.50 J/mol; in the powder with proportions of 40MDX:60SPS, the values ranged from 35007.14 to 38703.85 J/mol, in the powder with proportions of 20MDX:80SPS, the values ranged from 42116.12 to 46365.92 J/mol and in the powder with 0MDX:100SPS, the values ranged from 35679.64 to 39428.11 J/mol. The positive sign means that betalains degradation is a non-spontaneous reaction, and that it requires continuous energy supply (generally) or the presence of specific enzymes (catalysts) in order for the reaction to take place.
The activation enthalpy (ΔH#) is a measure of the energy barrier that must be overcome by the reacting molecules and is related to the strength of the bonds, which are broken and made in the formation of the transition state from the reactant (Vikram et al., 2005). Their values were similar for all of the evaluated temperatures in this study; in the beetroot juice, values between 2555.72 and 2356.18 J/mol were obtained, in the powder with 40MDX:60SPS, values between 3230.82 and 3031.28 J/mol were obtained, in the powder with 20MDX:80SPS, values between 2551.56 and 2352.03 J/mol were obtained and in the powder with 0MDX:100SPS, values from 3160.15 and 2960.61 J/mol were found. The positive sign for ΔH# represents an endothermic state, which means that there is heat absorption between the activated complex and the reactive one.
The activation entropy (ΔS#) measures the disorder change of the molecules in the system. The negative entropy values found in this study indicate that the transition state has lower structural freedom than the reactants and means that the system becomes more orderly and also confirms that it is an irreversible process. Table 3 presents the kinetic parameters obtained for some bioactive compounds from different authors, in the same way the determination of the thermodynamic parameters confirmed that the reaction is irreversible and non-spontaneous for the kinetic model based on the engineering of chemical reactions.
4. Conclusions
This study showed the kinetic degradation of betalains in beetroot juice, as well as in microencapsulated juices with different proportions of maltodextrin and sweet potato starch, considering the experimental conditions and computing level. A first-order kinetic model was used, and its parameters were estimated by the adjusted data in non-linear equations. In all cases, the results showed rapid degradation with low values for the activation energy; however, it is concluded that the microencapsulated juice with proportion 20 maltodextrin and 80 sweet potato starch presented the lowest value of activation energy and lower sensitivity to temperature, making the betalains more stable compared with the rest of samples. Additionally, the microencapsulated powder mentioned above had the highest retention of betalains. Finally, the measurement of thermodynamic parameters confirmed the previous assumptions of irreversible and non-spontaneous reactions for the kinetic model based on chemical reaction engineering. It could be recommended to study a broad range of temperatures and to extend the time of reaction, in order to obtain a stronger model able to predict the losses of betalains from another raw materials and microencapsulated under different conditions.