1. Introduction
Biotechnology is multidiscipline that allows us to manipulate living organisms from different techniques (Limera et al., 2017). During the last decades, biotechnology reached expressive advances in molecular and cellular biology, developing techniques that allow deepening in studies on physiological and genetic aspects of cells and microorganisms in in vitro culture (Currin et al., 2021; Giribaldi et al., 2021; Süntar et al., 2021). In plants, the use of molecular marker techniques to describe variations in plant genetics stands out, conserving DNA in genetic data banks (Stewart, 2008). However, tissue culture is essential for plant biotechnology, since it acts as an indispensable tool to produce genetically modified organisms (GMOs) (Cardoza, 2008). In addition, plant tissue culture presents a wider range of practical applications, which include: clonal propagation of elite varieties by apical buds; nodal segments; direct organogenesis and somatic embryogenesis; production of systemic disease-free plants by meristem culture; long-term conservation of endangered plants; induction of somaclonal variation by indirect organogenesis; obtention of haploids and doubled haploids by anther or microspore cultures; biosynthesis of secondary metabolites by cellular suspension; and somatic hybrids by protoplasts fusion (Anis & Ahmad, 2016).
In recent years, numerous studies have been published that used tissue cultures in food and medicinal species (Gulzar et al., 2020). In the area of food plants, the studies mainly address their objectives to the production of improved food, fiber, and fuels (El-Sherif, 2018), as well as the production of different morphologically and genetically stable fruiting herb cultivars (Naing et al., 2019). Nevertheless, some studies prioritized the effect of growth regulators on the micropropagation of tuber varieties (Hajare et al., 2021). On the other hand, in the field of medicinal plants, studies follow advances on the production of biologically active compounds (Espinosa-Leal et al., 2018), in vitro propagation, and the determination of the phytochemical and neuropharmacological profiles of Bacopa monnieri (Saha et al., 2020). Other studies acted on the in vitro propagation of Vaccinium vitis-idaea (Debnath & Arigundam, 2020) and the establishment of direct regeneration protocols for Cannabis sativa (Galán-Ávila et al., 2020). Likewise, in in vitro germplasm conservation, stem tips, nodal segments and embryogenic cultures have been used, both due to growth limitation at normal rates and growth limitation at minimum rates. However, in recent years significant progress has been made in conservation by total suppression of growth or cryopreservation of other types of explants such as microtubers, rhizome buds, roots, seeds, pollen or winter dormant buds (Ochatt et al., 2021; Wang et al., 2021).
The new University Law of Peru No 30220, promulgated on July 9, 2014 (El Peruano, 2014), indicates as University Social Responsibility (Article No 124) the following: "The ethical and effective management of the impact generated by the university in society due to the exercise of its functions: academic, research and extension services and participation in national development in its different levels and dimensions;… it is the foundation of university life, contributes to the sustainable development and well-being of society and engages the entire university community. " In this sense, the scientific and technological research developed in the university should be oriented to address and solve problems that arise in the sphere of influence of the university.
Although there are numerous studies on plant tissue culture, there are very few related to Peruvian species, especially in medicinal and forest species, but in some cases in food plants (Carrión & Tapia, 2019; Lapiz-Culqui et al., 2021). For this reason, the main objective of this research was to produce and release plants in vitro, using various techniques of plant biotechnology, specifically developed in the culture of plant tissues that are of agronomic, forestry, and medicinal importance.
2. Materials and methods
Collection of plant material, explants disinfestation and culture medium
The plant material used in the present study was of a di verse nature and were collected from different genetically isolated populations. The disinfestation processes were also different, although in most cases, ethyl alcohol and sodium hypochlorite were used as the main disinfectant (Table 1). The culture media used are detailed in Table 2. Additional characteristics of the plant material are summarised for each species used in this study.
Banana (Musa spp.). Musa L. spp; family Musaceae, cv. ‘Cavendish’, a dessert banana belonging to genomic group AAA, was collected from the field of the Centro de Formación Profesional Binacional - Mallares, Sullana, Peru, between the years 2008-2010. The germplasm was subsequently grown at the field of the University Campus of Universidad Nacional Pedro Ruiz Gallo (UNPRG), Lambayeque, Peru, and used in the present study.
Cassava (Manihot esculenta). Several varieties of M. esculenta Crantz, a native species to the South American tropics, were collected in many tropical regions throughout Peru during the years 1984-1990 (Flores-Meza et al., 2014). The germplasm was subsequently grown at the field ‘La Peña’ of UNPRG and transferred to the International Center for Tropical Agriculture (CIAT, Colombia) through meristem culture. Many varieties (MCol 22, MCol 33, MCol 2063, MCol 2215, MCol 2050, MCol 2032, MPer 441, MPer 443, MCub 49 and a local genotype) were released in several districts of Lambayeque and Cajamarca (Catache, Santa Cruz) (Delgado & Rojas, 1992). Recently, two native cassava varieties, both collected in the district of Salas (Pilasca), one of the poorest in the Lambayeque region, have been used to obtain healthy plants of systemic diseases through meristem culture and in vitro thermotherapy (30-35 oC for two weeks).
Matico (Piper tuberculatum). Spikes with mature seeds of P. tuberculatum were collected from along the shores of the Cumbil river (Lambayeque, Peru). Subsequently plants obtained by in vitro seeds culture were established in the gardens of the University Campus of the UNPRG. Currently, there appears to be a lack of plants of P. tuberculatum along in the Cumbil river due to floods and landslides caused by the “El Niño” phenomenon (ENSO/ El Niño-Souther Oscillation) of recent years (1982-1983, 1997-1998 and 2016-2017). The seeds, in number no more than 50, were disinfested in small bags of tulle to avoid their dispersion by water and disinfesting agents.
Montane forest species (Cedrela spp. and Dodonaea viscosa). Seeds of Spanish cedar (Cedrela odorata) and other cedar species (Cedrela montana, C. fissilis and C. molinensis), were collected from mature capsule in the process of dehiscence, to avoid dispersion of the seeds in the various districts of Lambayeque region (such as Kerguer and Pitipo), and La Capilla (Cutervo) in Cajamarca. In the case of chamana (Dodonaea viscosa, Sapindaceae), a shrub of significant importance for soil protection, the seeds were collected from mature fruits in the Santa Rosa de Sexi district (Santa Cruz, Cajamarca).
Pallar or Lima bean (Phaseolus lunatus). Pallar seeds of P. lunatus cv. Generoso de Ica (Ica-1548) and Sol de Ica (Ica-450) were supplied bt the Facultad de Agronomía of the Universidad Nacional San Luis Gonzaga de Ica (UNICA, Ica, Perú) and cultivated as starting material for in vitro cultures.
Peruvian brown cotton (Gossypium barbadense) and algodoncillo (G. raimondii). Seeds of Gossypium barbadense with various colors and tones of fiber (around 230 accessions) were collected in several districts of the Lambayeque, Piura, Cajamarca and La Libertad regions, and also in Amazonas and Ucayali regions. After removing the fibre, these seeds were cultivated in in vitro conditions for the establishment of the Germplasm Bank of Peruvian Brown Cotton in the UNPRG, by limitation the growth to minimum rates. Another important species is the algodoncillo (Gossypium raimondii), endemic to the coast and highlands of northern Peru and in serious danger of extinction. It is also being studied in in vitro cultures for large-scale propagation.
MS (Murashige & Skoog, 1962). The culture medium was supplemented with the following vitamins: 100 mg/L m-inositol and 1.0 mg/L thiamine. HCl and 0.7% agar-agar (pH = 5.8±0.1). PGR, plant growth regulators.
Pineapple (Ananas comosus). Crowns, arising from the upper part of the fruit, cv. Cayena Lisa and Golden, were obtained in the Moshoqueque Fruit Market of Lambayeque. The leaves were washed (with detergent and fungicide) and removed, before the disinfection treatment.
Pitahaya (Hylocereus megalanthus). Stem segments (ca. 50 cm in length) from adult plants, grown in Bagua Chica (Amazonas, Peru), were rooted and cultivated in a greenhouse, in pots containing a mixture of peat moss and soil (1:1) until they produced new joints.
Potato (Solanum tuberosum). The potato varieties and accessions were obtained from the International Potato Centre (Lima, Peru), and grown through in vitro cultures, specifically by meristem culture with the corresponding Phytosanitary Certificate. The plant material was in vitro multiplied and released to greenhouse conditions for the production of midi-tubers that were later distributed among the farmers of the Andean highlands of the Lambayeque (Kañaris) and Cajamarca (Cutervo and Llama) regions.
Rice (Oryza sativa). Seeds of O. sativa cv. Inti, Tallán and Viflor were obtained from the Instituto Nacional de Investigación Agraria (INIA, Lambayeque, Peru), and were manually dehusked, before the disinfection process.
Seasonally dry tropical forest species. Fruits and seeds of higuerón (Ficus spp. Moraceae) and hualtaco (Loxopterygium huasango, Anacardiaceae) were collected in the seasonally dry tropical forest of Lambayeque (Peru) and surrounding areas.
Sugarcane (Saccharum officinarum). The sugarcane varietes, namely, H-32-8560, H-37-1933, PCG-12-745 (Azul de Casagrande) and H-50, were obtained from the Empresa Agroindustrial Pucalá and Tumán (Lambayeque), and Caña Blanca and Caña Amarilla, from Pilasca (Salas, Peru). Stalk pieces were cut into nodal segments containing an axillary bud and sown in pots containing soil and sands and grown in a greenhouse. After four months, the plant material used for callus induction were the innermost layer of the leaves. These explants were cut into 5 mm pieces which were separately placed on an aseptic media (Jamil et al., 2017).
Sweet potato (Ipomoea batatas). Several varieties of I. batatas, a native species to the South American tropics, were collected in the Sweet Potato Germplasm Bank of the Instituto Nacional de Investigación Agraria (INIA, La Molina-Lima, Perú). The germplasm was subsequently grown at the field at ‘La Peña’ of the UNPRG and transferred to the International Potato Centre (CIP, Lima-Peru) through meristem culture.
3. Results and discussion
Table 3 shows the results in the production and release of plants of various species, as well as relevant complementary information.
Banana (Musa spp. cv. Cavendish (AAA). Shoot tip explants isolated from side shoots and disinfected by the sterilization process yielded 90% aseptic cultures with no fungal or bacterial contamination (Figure 1a). The two-step disinfestation, which involved treatment with ethanol followed with sodium hypochlorite solution, did not adversely affect the growth of cultures. After four weeks, the explants were removed from the initiation medium individually, sectioned vertically in two equal parts using a sharp blade, and placed onto a fresh multiplication medium. Subsequently, the axillary shoots which were 2 to 3 cm in height, were placed onto a rooting medium. During the disinfestation process it was not necessary to use mercuric chloride nor use a cocktail of antibiotics (rifampicin, kanamycin sulphate, and streptomycin sulphate) that were used by Agrawal et al. (2014), and which reportedly yielded 100% aseptic cultures. In the present work, the contamination rate was only 10% because the sword suckers were obtained from well-drained, sterilized soils. Side shoots with suspected contamination of Erwinia spp. (resulting in a fetid odor in the rhizome) were not collected for study. In Sullana (Piura), banana plants were produced in order to establish a series of axillary shoots using conventional methods, which were then distributed among the farmers of economically deprived resources throughout the region. In the University Campus of the UNPRG-Lambayeque, about ⁓50 plants were planted that were later used to propagate more shoots and are currently being disseminated across several locations: both within the University Campus itself, and in the Lambayeque region. The present study demonstrated the feasibility of using the plant tissue culture technique discussed here, to show the viable production of banana plants whilst still practicing the ethics of social responsibility within this field.
Musa is one of the most widely domesticated clonal genera grown across the tropics (De Langhe, 2010). They are usually propagated by vegetative means using asexual multiplication from side shoots, which are grown from lateral buds on the rhizome. However, this process is very slow with several negative impacts including transmission of diseases, pest epidemics, low production and poor preservation of the genetic material of the original plant that can affect the yield and quality of the eventual crop (Wambugu et al., 2008; Hussein, 2012). Our research has shown that the in vitro production of banana and other Musa is a very superior technology over traditional methods of production with respect to the optimal yield, uniformity, disease-resistant and keeping the planted material true to the type plants (Ngomuo et al., 2014).
Cassava (Manihot esculenta), several varieties. During the disinfestation process of the apical buds, a treatment with 70% ethanol and 0.5% sodium hypochlorite has been applied over a duration of three minutes, which was sufficient enough to reach a 100% disinfestation rate. This treatment did not adversely affect the growth of cultures, as 100% of the shoot tips were exhibited when grown in vitro on an initiation medium (4E culture medium) (Figure 1b). In other studies, treatments with 20% chlorox applied for 15 min were necessary to reach the maximum survival percentage of 75.6%, with a minimal contamination percentage of 19.8% (Abd Alla et al., 2013). In the present study, we used 0.3 mm high meristem culture with 2 leaf primordia, a sampled across a total of 278 cassava accessions transferred from the UNPRG to CIAT, including an additional 249 accessions introduced from the CIAT to UNPRG (Flores-Meza et al., 2014).
Table 3 Production of economically important plants species and released in the Lambayeque (Peru) region and surrounding regions
| No | Plant species/ var. or cv. | Lambayeque | Cajamarca | Piura | La Libertad |
| 1 | Banana (Musa sp.), cv. Cavendish (genomic AAA) | ⁓50 | - | ⁓2 500 | - |
| 2 | Cassava (Manihot esculenta), several varieties | - 278 accesions were transferred from UNPRG to CIAT and 249 accesions from CIAT to UNPRG - 10 varieties released in Pucala and other districts of Lambayeque - 02 varieties released in Pilasca (Salas) | - 10 varieties released in Catache (Santa Cruz) | - | - 10 varieties released in Pacanga and other districts of Chepén |
| 3 | Matico (Piper tuberculatum) | - ⁓700 plants | - ⁓300 plants | - | - |
| 4 | Montane forest species: Cedar (Cedrela spp.) and chamana (Dodonaea viscosa) | - 300 plants of several cedar species | - 700 plants of Spanish cedar and 700 plants of chamana | - | - |
| 5 | Pallar (Phaseolus lunatus), cv. Generoso de Ica (Ica-1548) | - 25 plants | - | - | - |
| 6 | Peruvian brown cotton (Gossypium barbadense), several accesions, and algodoncillo (G. raimondii) | - Several accesions (seeds) (~50) of G. barbadense and seeds of G. raimondii | |||
| 7 | Pineapple (Ananas comosus) cv. Cayena Lisa and Golden | - ~200 plants (cv. Cayena Lisa) and ~ 300 plants (cv. Golden) | |||
| 8 | Yellow Pitahaya (Hylocereus megalanthus) | - ~10 plants in greenhouse conditions | |||
| 9 | Potato (Solanum tuberosum), several varieties | - ~150 midi-tubers | - ~200 midi-tubers | ||
| 10 | Rice (Oryza sativa), cv. Inti and Viflor | - ~50 plants (somaclonal variants) | |||
| 11 | Seasonally dry forest species: Ficus spp. and Loxopterygium huasango | - ~50 plants (Ficus obtusifolia) and 15 plants (Loxopterygium huasango), in greenhouse conditions | |||
| 12 | Sugarcane (Saccharum officinarum), several varieties | - ⁓3,000 plants (somaclonal variants) | |||
| 13 | Sweet potato (Ipomoea batatas), several varieties | - ~400 (native accesions) for in vitro international transference |
Additionally, 10 varieties from CIAT were evaluated in Pucalá (Lambayeque) and Pacanga (La Libertad), reaching a total yield of 16.4 and 15.8 ton/ha, respectively. Local varieties, propagated using conventional methods, only reached a yield of 5.5 ton/ha (Delgado & Rojas, 1992), which was reached without the application of fertilizers or the use of pesticides. All this genetic material was subsequently propagated by stakes and distributed among the most financially deprived farmers within the region. Recently, 12 plants of two local varieties of cassava, which were free of pests or systemic diseases, have been donated to the community of Pilasca (Salas, Lambayeque) for large-scale multiplication by stakes.
Cassava (Manihot esculenta spp. esculenta; Euphorbiaceae) is native to the South American tropics and is closely related to M. esculenta spp. flabellifolia (Léotard et al., 2009). The importance of cassava as a food source and industrial crop relies on the rootstock, since they accumulate starch (approximately 30-60% dry matter), and therefore could be considered as the second most important source of starch globally. However, the average yield of cassava worldwide is only 12-13 tons/ha, but we have shown that its potential yield under optimal conditions can be as much as up to 80 tons/ha (FAO, 2013). That is why the application of biotechnology to control of viral infections, such as the cassava mosaic disease (CMD) and cassava brown streak disease (CBSD) (Legg et al., 2014), using in vitro propagation, synthetic seed application and other such technologies, can help to provide a biotechnological solution for the improvement of harvest cassava (Chavarriaga-Aguirre et al., 2016).
Matico (Piper tuberculatum). Hundreds of matico (P. tuberculatum) plants, obtained from in vitro germinated seeds (seedlings) and propagated by means of apical shoot and nodal segments (Figure 1c), were distributed across numerous districts of the Lambayeque and Cajamarca regions. These plants were successfully transferred to field conditions to produce inflorescence extracts with insecticidal potential. The locations were carefully selected to address especially the districts where there is a higher incidence of metaxenic disease such as malaria (transmitted by Anopheles sp.), dengue (transmitted by Aedes aegypti), and even uta (Leishmaniasis), transmitted by Lutzomya sp. (Delgado-Paredes et al., 2017).
Chemical studies carried out on Brazilian Piperaceae have revealed the occurrence of pyrones, lignoids and chromenes besides various amides bearing isobutyl, pyrrolidine, dihydropyridone and piperidine moieties (Parmar et al., 1997; Kato & Furlan, 2007). However, only lignans and amides compounds have demonstrated potential insecticidal and antifungal activity. One of the most widely studied species is P. tuberculatum, both for its antifungal potential (Da Silva et al., 2002; Palacios et al., 2009) as well as for its insecticidal potential (Soberón et al., 2006; Navickiene et al., 2007; Bazán-Calderón et al., 2012; Soberón et al., 2012; Mendoza-Frías et al., 2013; Monsalve-Asencio et al., 2015). P. tuberculatum colloquially known as ‘matico’, ‘nudillo’, ‘cordoncillo’ or ‘palo soldado’, is widely distributed from Brazil to Mexico.
Montane forest species: Spanish red cedar (Cedrela odorata, Meliaceae), and other cedar species, and chamana (Dodonaea viscosa, Sapindaceae). Around 700 individuals of Spanish red cedar (C. odorata) of about 30 cm high (Figures 1d and 1e); and 700 individuals of chamana (Dodonaea viscosa) of about 15 cm high, were planted in the towns of Sexi (Santa Cruz, Cajamarca) and Llama (Chota. Cajamarca). These two species, arboreal and shrubby in habit, respectively, will serve to protect the soil, which has been heavily eroded as the result of excessive deforestation combined with the increasing intensity of rainfall. Additionally, around 300 individuals of various species of cedar (C. odorata, C. montana, C. fissilis and C. molinensis) were planted in parks and avenues in various districts across the Lambayeque region. Currently, a reforestation program of about 10 hectares of ‘Spanish cedar’ (C. odorata), providing 1100 individuals per ha is being implemented in several locations in the Lambayeque and Cajamarca regions to combat further soil degradation.
As in many countries of Southern America, the establishment of hardwood plantations composed of C. odorata is limited by several factors such as the long-life cycle, their susceptibility to diseases and pest attack, and obtaining long-term economic credits (Pérez, 2006). However, an alternative to improve the economic profitability of the plantation is to establish it not exclusively for forestry, but rather find viable means of agro-forestry or even develop agro-silvo-pastoral systems (ASPS), where forage, leguminous and short-cycle vegetables are cultivated alongside the timber.
Our study suggests that in vitro germination not only makes it possible to achieve higher germination rates (95% to even 100%,), but also accelerate the reproduction by reducing germination time up to 60%. Additionally, using genetically resistant type material can help minimize the occurrence of bacterial and fungal diseases, both, superficial and endogenous. In in vitro cultures using clonal propagation of C. odorata, from node explants of the juvenile shoots, was reached using a MS basal medium supplemented with 2.0 mg/L-1 BAP and 3.0 mg/L-1 NAA.

Figure 1 Plant propagation and germplasm conservation. a. Musa sp. b. Manihot esculenta, c. Piper tuberculatum, d. Cedrela odorata (in vitro plants) and e. C. odorata (mass propagation).
This returned the best results of up to 100% shoot development with 3.93 cm mean height, showing no further morphological differences when compared to ex vitro seedlings (García-Gonzáles et al., 2011). However, the propagation rate using shoot apex or nodal segments will always be lower when compared with the propagation rate achieved with somatic embryogenesis, as was reported with the induction of indirect somatic embryogenesis from calli derived, immature zygotic embryos after three months of culture (Peña-Ramírez et al., 2011).
Pallar (Phaseolus lunatus). Only seeds of cv. Sol de Ica (Ica-450), established using in vitro culture, showed no contamination by bacteria and fungi and developed into viable aseptic seedlings. However, out of 100 seeds of cv. Generoso de Ica (Ica-1548), only one seed was aseptic while the rest showed a presence of Bacillus spp., a pathogenic endogenous bacterium (Figures 2a and 2b). The only aseptic in vitro plant obtained was established under greenhouse conditions and developed fruits and seeds with all normal characteristics. Of these aseptic seeds, 10 were randomly selected and cultivated again under in vitro conditions, obtaining seedlings all without the presence of Bacillus spp. bacteria.
While it is true that there are three major bacterial diseases of common blight (Xanthomonas campestris pv. phaseoli), halo blight (Pseudomonas syringae pv. phaseolicola), and bacterial brown spot (Pseudomonas syringae pv. syringae) (University of Illinois Extension, 2020), there are other groups of bacteria such as Bacillus spp. that severely affect the foliage, pods, and seedlings potentially causing great economic losses for farmers. As with other species such as rice or wheat, it is possible to select seeds that are free of bacterial and/or systemic fungi using in vitro culture since such pathogens can be visualized in a few days or weeks after application.
Peruvian brown cotton (Gossypium barbadense) and algodoncillo (G. raimondii). Seeds of G. barbadense, with dark brown, light orange and white fiber, were donated to the Regional Government of Lambayeque, for the establishment of two hectares of G. barbadense cultivation in the Íllimo locality (Lambayeque). These accessions also make up the Seed Germplasm Bank and the In vitro Germplasm Bank for G. barbadense (Figures 2c y 2d).
A strategy developed by the LGB-VRINV to disseminate and protect the cultivation of G. barbadense in the Lambayeque and surrounding regions is to deliver (exchange) small samples of the collected accessions of G. barbadense to farmers who still conserve sporadic culture samples. At present, most of these farmers have sown these accessions and now have a variety of fiber colors they did not have before, ensuring in some way the conservation of the genetic variation of the species (Delgado-Paredes et al., 2021a).
In the case of G. raimondii, after discovering the small locality of La Colmena (Chongoyape), which contains about three hectares of natural area and farmland and serves as the only and last refuge of this species in the Lambayeque region, the census of survival for the few existing individuals is by informing the local inhabitants to help maximize the conservational potential of the species in its natural environment (Delgado-Paredes et al., 2021b). Additionally, seeds collected in 2018 and 2019 were donated to the Seed Germplasm Bank and In vitro Germplasm Bank of G. raimondii (Figures 2e and 2f).
Several studies conducted in various places in the Americas have demonstrated the high degree of variability of G. barbadense genotypes. Therefore, we can assume there being considerable morphological and molecular diversity among the plants collected in the state of Amazonas, in contrast to those collected from the other states of Brazil (Hoffmann et al., 2018). In general, worldwide cotton germplasm collections exist in Australia, China, India, France, Pakistan, Turkey, Russia, United State of America and Uzbekistan (Abdurakhmonov, 2014), however, our Peruvian collections, especially those from the Universidad Nacional Pedro Ruiz Gallo de Lambayeque, feature the first seed germplasm bank and in vitro cultures of G. barbadense and G. raimondii.
Pineapple (Ananas comosus). Pineapple (A. comosus) var. ‘Cayena Lisa’ and ‘Golden’. Hundreds of pineapple plants (A. comosus, var. ‘Cayena Lisa’ and ‘Golden’) were produced and planted in different locations across the region of Lambayeque (Figure 2g).
Piña (the vernacular name in Peru) or pineapple is one of the most important, popular and delicious of American fruit in the world, and it is esteemed for its pronounced flavor and nutritive extracts containing vitamin A and B1 as well as a protein stimulating the digesting enzyme bromalain. Pineapple is propagated vegetatively through side shoots, slips or crowns taken from the fruit (D’Eeckenbrugge & Leal, 2003), the latter of which showing varying degrees of success (Khan et al., 2004). However, these planting methods have their limitations, including the potential transmission of diseases, less uniformity and inadequacy for commercial production (Mengesha et al., 2013). In Brazil, fusarium wilt (Fusarium subglutinans) is the most common, however, other problems that affect the commercial production negatively: such as the lack of high-quality propagules, low rate of multiplication of plants by conventional methods, and the lack of matrix, have severely limited pineapple production (Ruggiero et al., 1994). A similar situation occurs with the commercial cultivation of pineapple in Peru.
Yellow Pitahaya (Hylocereus megalanthus). The fruit of the yellow pitahaya is smaller than the red and pink pitahaya species (H. undatus, H. costaricensis, and other species from genera such as Selenicereus), and the white pulp contains numerous small edible seeds.
The commercial propagation of pitahaya is through stem segments (ca. 50 cm in length), which allows to reach fast vegetative growth and an early flowering and fruiting. Propagation via sexual reproduction is also another propagation form. When plants are grown from seed, the growth of the plants till a reproductive stage is very slow and can take up to seven years, additionally allowing a very high occurrence for genetic variability. That is why in vitro culture (Viñas et al., 2012; Hua et al., 2014; Montiel-Frausto et al., 2016), based on superior genotypes, enables large-scale clonal propagation to meet the growing demand of the commercial industry.
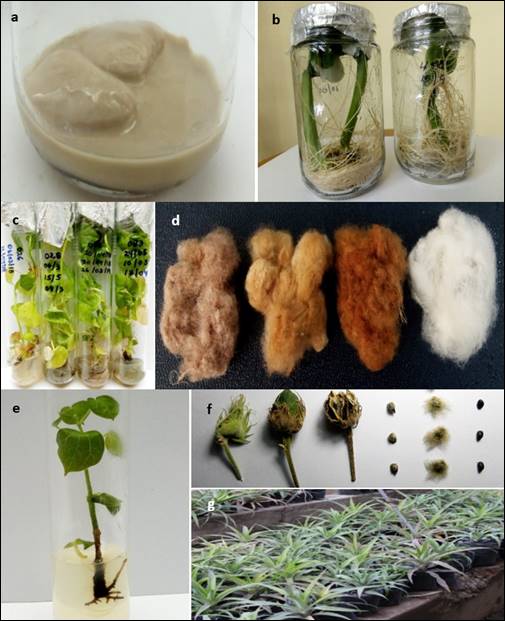
Figure 2 Plant propagation and germplasm conservation. a. Phaseolus lunatus (bacterial contamination), b. P. lunatus (healthy plants), c. Gossypium barbadense (in vitro plants), d. G. barbadense (several accesions), e. Plantlet propagated of Gossypium raimondii (algodoncillo), f. Fruits and seeds with green fibers of G. raimondii and g. Ananas comosus (mass propagation).
A new strategy for the propagation of pitahaya that has been implemented in our laboratory is to combine the in vitro propagation using higher genotypes (cloning) (Figure 3a) followed by the cross-fertilization of in vitro plants under greenhouse conditions, to produce fruits and seeds sexually using crosspollination. This method would allow thousands of ‘sexually cloned’ individuals to be obtained, without creating random variability as the result of crosspollination with lesser genotypic plants grown in its vicinity.
Potato (Solanum tuberosum). During the April till October 2017 and 2019, when the night temperature range between 17 to 19 oC, the induction of midi-tubers of potato grown in greenhouse conditions over a period of 3 to 4 months, were obtained (Figure 3b). Around 500 midi-tubers, ranging in size between 5 to 20 mm in length were sampled. When the ‘agostamiento’ (dry out) was over, they were distributed among potato farmers of the Andean regions of Lambayeque (Kañaris), Cutervo and Llama (Cajamarca). The following varieties of potato were used for this study: Amarilis, Atlantis, Canchán, Capirona, Perricholi, Tacna, Única and Yungay. The genetic material was free of pests and systemic diseases and were used as foundation for mother plants to increase the yield in regions where phytosanitary factors are very limiting. Kañaris is a poor locality in Lambayeque where resources are scarce.

Figure 3 Plant propagation and germplasm conservation. a. Clonal propagation of Hylocereus megalanthus, b. Midi-tubers of several varieties of potato, c. Somaclonal variants of rice, d. Germplasm conservation of Ficus sp. e. Somaclonal variants of sugarcane and f. In vitro propagation of sweet potato.
Since the great famine in Ireland that killed more than a million people due to Phytophthora infestans (Late Blight) infestation on potato crops (Callaway, 2013), there have been many other diseases and infestation identified that affect potato tubers in various proportions. These diseases are caused by bacterial, fungal and viral pathogens such as Streptomyces spp. (Common Scab), Alternaria solani (Early blight), Fusarium spp. (Fusarium Dry Rot), Colletotrichum coccodes (Black Dot), Helmintosporium solani (Silver Scurf), Rhizoctonia solani (Black Scurf and Rhizoctonia Canker), Phytophthora erythroseptica (Pink Rot), Phythium spp. (Phytium Leak), Phytophthora infestans (Late Blight). Other viruses that have been found are broadly classified as Potato Virus Y, Potato Virus X, Potato Virus S, and the PSTV viroid (Potato spindle tuber viroid) (Singh, 2014; Scheufele, 2016; Kreuze et al., 2020).
For their susceptibility to viral infections, it utmost important to obtain plants thar are free of pests and systemic diseases. One of the mostly applied techniques for propagation is the meristem culture, which could be used in combination with other techniques such as thermoterapy, chemoterapy, electroterapy or cryotherapy.
Rice (Oryza sativa). There are around 35 somaclonal variants of rice cultivars, of the varieties Tallán and Viflor, obtained in callus induction and plant regeneration placed in a culture medium (indirect organogenesis) (Figure 3c). These were subsequently cultivated under saline conditions and thermal stress (low temperature) showing that some of these genetic variants exhibet greater adaptability to such conditions (Delgado-Paredes et al., 2013). Likewise, about 250 somaclonal variants of rice cultivars, from the varieties Inti and Viflor, were obtained by indirect organogenesis under saline conditions (0.0 to 1.5% NaCl), which showed similar adaptability in these somaclonal variants to stressful salinity conditions of up to 1.5% (Mori-Gastelo et al., 2015).
Somaclonal variation is an effective term proposed by Larkin & Scowcroft (1981), to refer to the genetic and physiological changes observed as a result of in vitro plants cultures. Such genetic and epigenetic changes, usually observed as a result of indirect organogenesis (with previous callus formation) are very useful as a new and alternative tool for obtained genetic variability in short time periods and without complex technological mechanisms. However, this technique becomes more difficult when it is highly desirable to produce true-to-type individuals (Krishna et al., 2016). In the case of rice, due to the high natural genetic uniformity index, resulting from self-fertilization, somaclonal variation is a very important mechanism for inducing genetic variability.
Seasonally dry tropical forest species. It has been estimated that around 300 woody species of trees and shrubs exist in the seasonally dry tropical forest (SDTF) of Peru and Ecuador. With the exception of the Fabaceae family, it is possible that most of the other species deliver seeds with recalcitrant physiological behavior. That is why studies have been initiated on the conservation of seeds and in vitro cultures of two species which portray a greater relevance of the SDTF habitat: higuerón (Ficus spp.) (Figure 3d) and hualtaco (Loxopterygium huasango). Higuerón is important for its great arboreal coverage, shown to be one the most valuable forest assets in studies on biomass and carbon sequestration. Hualtaco has historically been excessively logged for its commercially valuable timber. In both species, in vitro germination has allowed to obtain up to 100% germination from seed thar are grown within three months from collection, which has produced several dozens of saplings for reforestation and forest restoration purposes within their natural environment. Under normal greenhouse conditions, the seed germination rate was less than 20%.
In vitro plant tissue culture has been widely used in seed germination and induction of various morphogenic processes of Ficus species such as fig (F. carica) (Singh et al., 2016), F. benjamina (Lavanyad et al., 2006), and F. religiosa (Siwach & Gill, 2014). Studies to the seed viability oh hualtaco has been more limited, only represented by a single study conducted by Conde et al. (2017) of the Universidad Nacional de Loja (Ecuador) of which the results are mostly preliminary.
Sugarcane (Saccharum officinarum). For this study, sugarcane varieties were provided by EA Tumán, and about 3,000 individuals were obtained by indirect somatic organogenesis, a process that led to the production of plants with somaclonal variation (Figure 3e). A selection of the 50 most vigorous individuals were isolated, selected on characteristics including: height and plant diameter, number of tillers, tolerance to salinity and drought, and percentage of harvestable sucrose. This selection was again cultivated over two periods of 14 and 16 months. A somaclonal variant of var. H-50 doubled the content of tillers (from 20 to 40) and the sucrose content increased by 0.5% from the average obtained across the F1 crop. Since 1990, these somaclonal variants are being planted in the agricultural fields of EA Tumán. The importance of somaclonal variation, as a conventional biotechnological tool, has been widely discussed by Krishna et al. (2016).
Sweet potato (Ipomoea batatas). Around 400 sweet potato accessions (IN, Native Introduction), were transferred to the International Potato Centre (CIP), through meristem cultivation, which produced an average of 2-5 test tubes per accession (Figure 3f). Subsequently, some accessions of this genetic material were transferred by the International Potato Center to the United States Department of Agriculture in Beltsville, Maryland.
Since the 1970s, meristem culture has been used to obtain sweet potato plants free of pests and systemic diseases caused by viral, fungal and bacterial infection. This trend has not changed in recent years, as evidenced by studies conducted by Wondimu et al. (2012) in Ethiopia, and Alam et al. (2013) in Bangladesh. It is certain that the use of this biotechnological tool has allowed to significantly reduce the spread of systemic sweet potato diseases worldwide, and improvement can be sought on how to make this process even more efficient across sweet potato and other tuberous species.
4. Conclusions
The various techniques of in vitro plant tissue culture have been applied in the obtention of plants free of systemic diseases, micropropagation, genetic improvement, germplasm conservation and induction of secondary metabolites. All these techniques have played an important role of social responsibility in many parts of the world, especially in countries with lower economic resources, allowing them to attend to their basic needs. That is why at UNPRG-Lambayeque, through the General Biotechnology Laboratory (LGB) and various collaborators from organizations both nationally and internationally, these activities are being carried out and improved gradually. Peru is a megadiverse country which has produced numerous food plant species, which will hardly allow the entry of genetically modified organisms (GMO). In addition, GMO crops have not solved the serious problem of hunger and malnutrition currently facing the world population. For all this, this study has emphasized in the use of in vitro culture techniques and the refinement of various protocols, to contribute to the development of agriculture, the protection of forest species and the use of medicinal plants of the region and the country. Certainly, the application of more advanced technologies will make it possible to explore other fields of plant biotechnology such as the induction of haploids, the obtaining of protoplasts and the production of secondary metabolites.