1. Introduction
In Peru, the Anacardiaceae family is recognized for having 13 genera and 40 species (Brako & Zarucchi, 1993; Ulloa et al., 2004), among trees and shrubs. Seven endemic species are classified in three genera including Orthopterygium, an endemism in the level of genera (León & Monsalve, 2006). The hualtaco (Loxopterygium huasango Spruce ex Engl.) Mauria heterophylla Kunth., M. membranifolia Barfod & Holm-Nielsen, M. suaveolens Poepp., Schinus molle L. and Spondias purpurea L., constitute the six species of woody Anacardiaceae of seasonally dry forests or seasonally dry tropical forests (SDTF). This forest extends through the northwest of Peru, occupying the regions of Tumbes, Piura, Cajamarca, La Libertad and Lambayeque, and L. huasango is distributed from 200 to 1500 masl and continues to the south of Ecuador, occupying the provinces of Loja and Guayas, distributed from 0 to 700 masl (Aguirre et al., 2006). Although these authors did not include L. huasango for the region of Lambayeque, nevertheless constituting one of the most important species of deciduous dry forest and forest dry semideciduous of this region, such as that was reported in a study carried out in ants from Reserva de Vida Silvestre Laquipampa (Castro et al., 2008) and in the Reserva Ecológica (Lambayeque), where it even reached the second place in the Importance Value Index (IVI) (Linares-Palomino & Ponce-Álvarez, 2009).
L. huasango, a critically endangered desert plant from Peru, has been described as a unarmed, resinous, deciduous trees, 5 to 18 m higth, cylindrical shaft and also irregular and tortuous; 20 to 60 cm in diameter, globose arboreal cup, sympodial branch and foliage greenish-yellow; white, colored and black wood, exuding droplets of whitish and sticky resin; the outer bark is cracked and light brown and the inner bark is homogeneous and whitish; the imparipinnate leaves are impacted, alternate, spirally arranged, 12-22 cm long, 2-4 pairs of leaflets opposite, crenate or serrate, 6-9 cm long and 1-3 cm wide; with inflorescences an axillary panicle, flowers hypogynous, unisexual, plant dioecious; sepals 5, imbricate; petals 5, imbricate; male flowers with 5 stamens; nectary-disk intra-staminal, annular, 5-lobed and female flowers with superior ovary keeled, 1-locular, 1-ovulate with 3 lateral excentric styles; male flowers with vestigial ovary, female flowers with vestigial stamens; fruit a dry, endocarp bony, with winglike coriaceous membrane, 1.5 cm long and 5-6 mm wide, brownish white and cream-colored irregular triangle shaped seeds (Pennington et al., 2004; Centro IDEAS, 2006; Reynel et al., 2006; Carrillo, 2015). Additionally, L. huasango forms a root system with great capacity to develop on rocky, compact, and extremely dry slopes, spreading through the cavities of the rocks until reaching flat areas (Centro IDEAS, 2006) and at flowering time the leaves produce stinging hairs which cause hives on contact with the skin, painful in allergic people (Carrillo, 2015), so the local inhabitants that the L. huasango ‘urine’ or ‘micciona’.
L. huasango has been scarcely studied in forestry. Pioneering studies performed by Aróstegui (1976) who determined the physical and mechanical properties of wood, and Chavesta (2005) who described the anatomy of wood and its possible uses; however, L. huasango has different uses. As a forest species, wood is used in the parquet industry and for the manufacture of chairs, cots and tables, while the rural population uses it for the manufacture of thresholds, beams, doors, windows and fence posts due to its high resistance to humidity (Centro IDEAS, 2006), since the heartwood is resistant to the attack of xylophagous fungi although the sapwood is susceptible to the attack of biological organisms, due to the drying of the wood to follow a slow process to avoid deformations (Chavesta, 2005). Also, the resin is used for rubs in case of dislocations, rheumatic and muscular pains, anesthetic and extraction of decayed teeth, while healers use it in their magical practices and as a repellent; the foliage is also used as fodder (Centro IDEAS, 2006; Carrillo, 2015); however, it is important to recognize that L. huasango, as well as the ‘guayacán amarillo’ [Tabebuia chrysantha = Handroanthus chrysanthus (Jacq.) S.O. Grose] and the ‘guayacán negro’ [T. billbergii = H. billbergii (Bureau & K. Schum.) Standl.], two other emblematic SDTF species, were widely used in the decades from 60 to 80, in the manufacture of parquet, decreasing significantly in subsequent decades due to overexploitation of the resource and the advent of ceramics and porcelain. For all these reasons Reynel et al. (2006) considered more than a decade ago that the populations of this species are very restricted and localized, being in danger in the country so it is important to spread this propagate this valuable species to prevent its eradication. Unfortunately, not only the rural inhabitants carry out the illegal activity of extracting firewood and wood without authorization, but also the military barracks in the area, for construction, parquet, firewood, and coal (Leal-Pinedo & Linares-Palomino, 2005).
In such a way, a study conducted at the SDTF Chililique (Chulucanas, Piura), according to Peruvian law D.S. 043-2006-AG, with an altitudinal range of 600 to 950 meters above sea level and a small area of 128.6 hectares, two endemic species, Psidium rutidocarpum Ruiz & Pav. (‘guayaba de monte’/Myrtaceae) and Caesalpinia pai pai (= Libidibia glabrata (Kunth.) C. Cast. & G.P. Lewis) (‘charán’/Fabaceae) and five Critically Endangered (CR) species, Bursera graveolens (Kunth) Triana & Planch. (‘palo santo’/Burseraceae), Colicodendron scabridum (Kunth) Seem. (‘zapote’/Capparaceae), Capparis eucalyptifolia O.L. Haught (‘frejolillo’/Capparaceae), Celtis iguanaea (Jacq.) Sarg. (‘palo blanco’/Cannabaceae) were reported, in addition to L. huasango; species that are being heavily exploited locally and require a management plan to avoid decimating their populations (Carrillo, 2015). Likewise, Vilela (2018), studying asexual propagation, used stakes of L. huasango from the SDTF from Lancones (Sullana, Peru), 30 cm long and one cm in diameter, 500 ppm indole butyric acid (Rapid root) and substrate with goat manure 50% more agricultural land 50%, obtained the highest production of seedlings consisting of 1 667/20 m2 and the highest benefit/cost ratio of 2.66.
Among the ecological studies that highlight the presence of L. huasango in the SDTF of Ecuador and Peru we have the one on the floristic composition and state of conservation of the SDTF of south-western Ecuador, in which only in the dense deciduous forest L. huasango was reported, has forming populations with more than 800 individuals/ha of 15-18 m height and dbh ≥ 5.0 cm (Aguirre & Kvist, 2005). In addition to that study, Aguirre et al. (2006) pointed out that among the vegetal formations of the SDTF in the Tumbesina Region: there is the dry thorny scrub, the deciduous forest and the semideciduous forest, such that only in the deciduous forest L. huasango is considered a characteristic species in the structure of the vegetation consisting of aparasolados and thorny trees up to 15 m high, although it is not mentioned for the semideciduous forest but it is known that in the SDTF of Peru there are still numerous specimens of L. huasango. Additionally, a study conducted in the Reserva de Biosfera del Noroeste (Peru) determined that L. huasango was in the group of species with densities between 8.81 and 1.50 trees/ha, in the upper diameter classes (dbh > 30 cm) (Leal-Pinedo & Linares-Palomino, 2005). In the classification of the vegetation in seasonal ecosystems, by means of the quantitative analysis UPGMA (Unweighted Pair Group with Arithmetic Mean) and MDS (Multidimensional Scaling), using the dissimilarity index Bray-Curtis (or Sørensen quantitative) as a measure in the MDS, from the dry forests of Piura, was determined that among the 65 plots evaluated, L. huasango was one of the three most frequent species, registering in 35 plots, while in the six groups UPGMA (A-F), L. huasango was one of the most characteristic species in groups B, D, E and F, from 100 to 750 masl (La Torre-Cuadros & Linares-Palomino, 2008). Otherwise, the importance of L. huasango was evidenced in the evaluation carried out in the SDTF La Menta (Piura) where the basal area, for the evaluated area, was 128.86 m2, highlighting L. huasango with 45.97 m2, while the IVI was 43.0% for only three registered individuals (Rasal et al., 2011). In addition, a study performed on the phenology of some species that are food for the white-winged guan (Penelope albipennis Taczanowski), a endemic bird seriously threatened, in the Reserva Ecológica Privada Chaparrí, Chongoyape (Lambayeque), between June 2004 to July 2005, it was determined that in L. huasango trees of 8 m height, the vegetative development occurred between January to July with a maximum of 82.8% in January, with the highest buds formation (40%) in December, while the flowering occurred between January to march with a maximum of 17.5% in January and fruiting occurred between march to May with a maximum of 10.9%, between green and ripe fruits (Martos et al., 2009).
Several factors including genotype, macro- and micronutrients, plant growth regulators, explant type, pre-treatments, culture conditions, culture vessels, and phenolic secretion have shown to influence in vitro plant regeneration and plant propagation of several species. Plants propagation through axillary shoot bud activation are more reliable clones than the plants regenerated through callus differentiation, since the chances of somaclonal variations are more in plants regenerated through callus differentiation then axillary shoots (Paliwal et al., 2018). The first study about in vitro propagation of L. huasango were reported by Millones (1995) and Delgado-Paredes et al. (2021) who preliminarily studied aspects related to germination of seeds in MS culture medium supplemented with various concentrations of gibberellic acid (GA3), clonal propagation from apical shoots and nodal segments, plantlets obtained from germinated seeds, the induction of calluses in various concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D) and regenerated roots, as the only morphogenic response, in the cytokinins BAP, KIN and 2iP, supplemented individually or in interaction with the auxin AIA. While a study carried out in in vitro cultures of ‘hualtaco’ (L. huasango), collected in the SDTF of Loja (Ecuador), seeds germination was evaluated in culture medium supplemented with GA3, and clonal propagation with the cytokinin 2iP, adenine sulfate and coconut water (Conde et al., 2017). But not only in L. huasango studies in tissue culture of SDTF species are scarce but also in other species of this fragile ecosystem. Recently only two studies were published, one on ‘cerecillo’ (Muntingia calabura L.) (Duque-Aurazo et al., 2020) and ‘faique’ [Vachellia macracantha (Humb. & Bonpl. ex Willd.) Seigler & Ebinger] (Sosa-Amay et al., 2020).
The aim of this study was to evaluate seed germination, callus induction, clonal propagation, and in vitro germplasm conservation of hualtaco (Loxopterygium huasango), one of the most representative species of the seasonally dry tropical forest of northwestern Peru, with the purpose of conserving the most valuable genotypes and tending to propagate them on a large scale in reforestation programs.
2. Materials and methods
Plant material
The collection of hualtaco (Loxopterygium huasango Spruce ex Engl.) was carried out in several places in the seasonally dry tropical forest of Lambayeque, specifically on the Olmos - Jaén (Portachuelo de Olmos) (UTM: 17M 644912E 9335622S) road and Pilasca (Salas) (UTM 17M 655668E 9310341S), from the Lambayeque region (Peru). Only in Pilasca the fruits were collected from the soil (Figure 1). The plant material was collected from the adult tree branches, greater than 12 m in height and straight stem, between the months of April to July of 2017 to 2019 (Figures 2a, b, c) and was made up of mature fruits type samara (Figure 3a).
Plant material preparation and seed disinfestation
The largest fruits were selected, under the best phytosanitary conditions and with optimal morphological characteristics, to which the pericarp was removed using clamp and scalpel No 10 and a binocular stereoscope, trying to eliminate all remaining pericarp, potential source of exogenous contamination. The seeds were washed with detergent for 10 min and then grouped in several 25 in small bags of tulle for disinfestation, in horizontal laminar flow chamber. These were pretreated with 70% (v/v) ethanol for 60 s and surface-sterilized with commercial sodium hypochlorite (bleach Clorox®, 5% active chlorine), in a 1:1 ratio with distilled water, for 10 min, followed by 3-5 washes with sterile water. The excised seeds were subsequently inoculated onto the seed germination MS (Murashige & Skoog, 1962) medium supplemented with 0.0, 0.5, 2.5 and 5.0 mg/L GA3, contained in 130x12 mm test tubes, at a rate of two seeds for test tube, making a total of 50 seeds for treatment, one week after being collected.
Callus induction and morphogenic process
In the callus induction, several explants (seeds, cotyledons, hypocotyls, and roots), isolated from 30-day-old seed germinated in vitro, were used. The MS medium was supplemented with 0.0 to 5.0 mg/L-1 2,4-D, and the explants were sections of cotyledons (0.5 mm2), hypocotyls and roots (10 mm long), as well as whole seeds, inoculated into flasks of 80x50 mm, at a rate of three explants/flask. In an additional experiment, for morphogenesis of in vitro-raised cotyledon, hypocotyl and root explants, MS medium supplemented with 0.2 mg/L-1 IAA and 1.0, 2.0 or 4.0 mg/L-1 BAP was used. Each treatment consisted of 15 explants and all treatments were repeated twice. The asepsis of the explants was considered per se.
Clonal propagation and in vitro germplasm conservation
In clonal propagation, cotyledonary nodes of in vitro cultured seedlings from 30-days-old, formed by shoot apex of 0.5 cm, two cotyledons and hypocotyl section (2 cm length), were inoculated onto MS medium containing 0.02 mg/L-1 IAA or 0.02 mg/L-1 NAA or 0.02 mg/L-1 IBA with 0.02 mg/L-1 GA3, respectively, for activation of shoot buds. These explants were inoculated in 150x18 mm test tubes. Data on shoot elongation were recorded every two months for six months. Each treatment consisted of 10 explants and all the treatments were repeated twice.
In an additional experiment, cotyledonaray node explants were used for multiple shoot induction and proliferation. The explants had the same morphological characteristics as those used in the clonal propagation experiments. These explants were inoculated onto MS medium supplemented with 0.5, 1.0 and 2.0 mg/L-1 of cytokinins BAP, KIN or 2iP, respectively. These explants were also inoculated into 150x18 mm test tubes. Data on shoot elongation were recorded every month for three months. Each treatment consisted of 10 explants and all the treatments were repeated twice.
Re-growth on in vitro germplasm conservation
In in vitro germplasm conservation, the same plants propagated in clonal propagation treatments were evaluated after nine months of in vitro growth showing a normal phenotype. Subsequently, 50% of these apical shoots and nodal segments were cultured in culture medium supplemented with 0.02 mg/L-1 IAA 0.02 mg/L-1 GA3, and the other 50% in culture medium supplemented with 2.0 mg/L-1 AgNO3 in 150x25 mm test tubes. Data on shoots elongation were recorded every month for three months. Each treatment consisted of 25 explants and all the treatments were repeated twice.
Culture medium and culture conditions
All the culture media incorporated the mineral salts MS, the vitamins m-inositol 100 mg/L-1 and thiamin.HCl 1.0 mg/L-1 and 20 g/L-1 sucrose, which were supplemented with various growth regulators, depending on the induced morphogenic process.
The pH was adjusted to 5.8 ± 0.1 with HCl (1 N) or 0.1N KOH (1 N) before gelation with 0.6% (w/v) agar-agar. Sterilization was performed in a vertical autoclave for 20 min at 121 oC and 15 lbs/in2 pressure. The culture seeds and callus induction were incubated in the dark for 4 and 8 weeks, respectively; for morphogenic responses, clonal propagation, and in vitro germplasm conservation for 2, 6 and 9 months, respectively, and the light intensity was 50-70 μmol m-2 s-1 on a 16-h photoperiod provided by cool-white, fluorescent lamps. The cultures were incubated at 25 ± 2 oC.
Ex vitro seed germination
A group of 50 seeds, without pericarp, collected from trees of Portachuelo de Olmos, were soaked in distilled water for 48 hours and then cultured semi-buried into pots (10 cm in diameter) containing a 2:1 (v/v) soil mixture of humus: river sand under greenhouse conditions (13/11 h, light/dark and 26 ± 2 oC). The treatments were repeated twice.
3. Results and discussion
Seed germination
Only the seeds collected in Portachuelo de Olmos trees and in vitro cultivated one week after the collection, reached germination rates between 70 to 90%, being higher as the GA3 concentration increased from 0.1 to 5.0 mg/L, whereas in the control treatment, lacking GA3, the germination was not greater than 10% (Table 1). In the most vigorous seeds germination began at 7 days with the emergence of the radicle and continued until day 30 of the crop, reaching an elongation of the hypocotyl of 1.5 to 2.8 cm in height, with the cotyledons well expanded and 2-3 main roots with numerous lateral roots and an intense dark purple color (Table 1,Figure 3b), although the caulinar apex started the growth after 30 days; however, with these morphological characteristics the seedlings can be transferred to soil conditions in the greenhouse, if the purpose is clonal propagation from sexual seed. In the only disinfestation treatment tested, with commercial sodium hypochlorite (Clorox® bleach, 5% active chlorine), in a 1: 1 ratio with distilled water, for 10 min, the contamination rate was 5%.
Table 1 In vitro seed germination of L. huasango, collected from Portachuelo de Olmos, after 30 days of culture
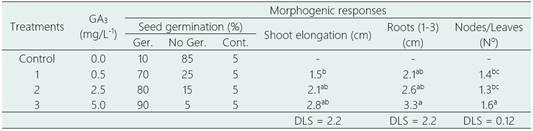
Ger: germination; Cont.: contamination.
The seeds collected in the soil, in the Pilasca SDTF, despite having been selected with the help of the stereomicroscope, only 5% germinated, 15% did not germinated and 80% were contaminated with fungi. Most of the seeds collected were seriously damaged by insect attack.
In the study carried out in the seeds culture of L. huasango, collected in the Loja SDF, the contamination rate was between 0 (50% NaOCl for 5 min immersion) to 35% (50% NaOCl for 15 min immersion), very contradictory results given that the greater the time of immersion, the disinfestation percentage must have been higher (Conde et al., 2017). Likewise, in this study it is not mentioned whether the pericarp, the main source of contamination, was removed from the seed. Regarding the germination rate, it was reported that it was ⁓78% in seeds without scarification and in culture medium supplemented with 1.0 mg/L-1 GA3 21, a result very similar to that obtained in the present study.
Callus induction
The process of callus induction was initiated at 30 days of the culture reaching the highest percentages (80 to 100%) in the explants of whole seeds and cotyledons, in treatments tested with 2,4-D (0.1 to 5,0 mg/L-1), 90% in hypocotyls in treatments with 3,0 to 5,0 mg/L-1 2,4-D and 100% in roots in treatments with 4,0 and 5, 0 mg/L-1 2,4-D (Table 2).
Table 2 Callus induction from several explants of L. huasango, after 45 days of culture
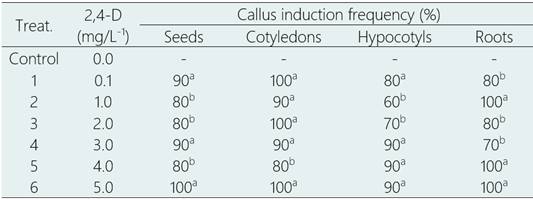
Values within a column followed by a different letter are significantly different at P < 0.05 according to Tukey’s multiple range test.
In table 3 it is observed that among all the explants used, in cotyledons the highest frequency was reached with 83.89%, in the 45 days after culture with 87.08% and in treatment with 5.0 mg/L-1 2,4-D with 80.00%. However, in the interaction of the following factors: explant type - culture time - growth regulator concentration, the combination cotyledons - 45 days - 1.0 mg/L-1 2,4-D, it was the most relevant.
Table 3 Effect of several factors (type of explants, exposure time, growth regulator concentration and interaction between these factors, in callus induction of L. huasango, after 45 days of culture
| I. Factor | ||
| Order of Merit (OM) | Explants type (E) | Callus formation (%) |
| 1 | Cotyledons | 83.89a |
| 2 | Seeds | 65.56b |
| 3 | Hypocotyls | 62.78b |
| 4 | Roots | 61.67b |
| DLS = 7.9 | ||
| II. Factor | ||
| OM | Culture time (days) (T) | Callus formation (%) |
| 1 | 30 | 74.58ª |
| 2 | 45 | 87.08ª |
| 3 | 15 | 43.75b |
| DLS = 6.3 | ||
| III. Factor | ||
| OM | Concentration of plant growth regulator (mg/L-1 2,4-D) (C) | Callus formation (%) |
| 1 | T6 (5.0) | 80.00a |
| 2 | T5 (4.0) | 69.17ª |
| 3 | T3 (2.0) | 68.33ª |
| 4 | T1 (0.1) | 65.33ª |
| 5 | T2 (1.0) | 65.00a |
| 6 | T4 (3.0) | 62.50ª |
| DLS = 10.8 | ||
| IV. Factor | ||
| OM | Interactions: Explants - Time - Concentrations | Callus formation (%) |
| 1 | Cotyledons - 45 d - 1.0 | 100.00a |
| 2 | Cotyledons - 45 d - 4.0 | 100.00a |
| 3 | Cotyledons - 45 d - 5.0 | 100.00a |
| 4 | Hypocotyls - 30 d - 0.1 | 100.00a |
| 5 | Hypocotyls - 30 d - 5.0 | 100.00a |
| 6 | Hypocotyls - 45 d - 0.1 | 100.00a |
| 7 | Hypocotyls - 45 d - 2.0 | 100.00a |
| 8 | Hypocotyls - 45 d - 5.0 | 100.00a |
| 9 | Roots - 45 d - 5.0 | 100.00a |
| 10 | Seeds - 45 d - 3.0 | 90.00b |
| 11 - 19 | … | … |
| 20 | Roots - 45 d - 3.0 | 90.00b |
| 21 - 71 | … | … |
| 72 | Cotyledons - 15 d - 0.1 | 10.00d |
| DLS = 46.8 |
Values within a column followed by a different letter are significantly different at P<0.05 according to Tukey’s multiple range test.
The callus was dark purple and highly friable, covering the explant slightly, so they can be used in the establishment of cell suspensions (Figures 3c and 3d). 2,4-D, a potent synthetic auxin widely used as herbicide, has also been used in callus induction in numerous plant species.
Thus, the optimum callus induction, from bulb scales of Lilium mackliniae Sealy, a rare endangered Asiatic lily species, was obtained with 2.0 mg/L-1 2,4-D (Sahoo et al., 2019). In the establishment of Agrobacterium-mediated transformation of Paspalum vaginatum, sterilized seeds were placed on MS basal medium supplemented with 2.5 mg/L-1 2,4-D for callus induction (Wu et al., 2018). Likewise, callus cultures were established from nodal explants of several chemotype of Plumbago zeylanica L. on MS medium augmented with 0.1 to 10 μM 2,4-D (Sharma & Agrawal, 2018). In Passiflora cincinnata embryogenic calli were induced in another culture in medium with various concentrations of 2,4-D and BA (da Silva et al., 2021). In another study, the tuber of Rhizoma zedoriae [= Curcuma zedoriae (Christm.) Roscoe] were sliced and then transferred to solid MA medium to induce callus with 2.0 mg/L-1 2,4-D and 0.2 mg/L 6-BA (Wang & Wang, 2019).
Another demonstration of the importance of callus, as a tool in plant breeding and biotechnology, was observed in selected genotypes of Triticum aestivum where the induction and regenerative capacity of callus was stimulated by lipopolysaccharides of various races of Azospirillum (Tkachenko et al., 2021), as well as the large-scale regeneration of hermaphrodite emblings of Carica papaya using early molecular sex determination during embryogenic callus multiplication (Xavier et al., 2021). In all these studies, as in the study presented, several concentrations of 2,4-D induced up to 100% of callus induction, depending on the explant used. However, other auxin-cytokinin formulations can induce callus formation as has recently been observed in in vitro culture of Vitis vinifera L. rootstocks (Gutierrez-Rosati & Gonzales, 2019).
Morphogenic responses
The only morphogenic response observed in callus of cotyledons and hypocotyls from L. huasango was the roots induction forming a profuse root system with a frequency of 10.8 a 13.8% in all the treatments of IAA, IAA-BAP, IAA-KIN or IAA-2iP, after 45 days of culture (Table 4, Figure 3d). The response was similar, both in cotyledons and hypocotyls callus, and in no treatment the shoots formation was induced. These roots can be used in mass root culture systems with the purpose of secondary metabolites formation of economic interest.
In other experiments with IAA-BAP, in some treatments only friable callus formation was observed and in others the roots formation, specifically when the explants were roots (Table 5).

Figure 3 L. huasango: a. Samara fruits and seeds: b. Seedlings with 15-d-old, c. Callus induction and roots regeneration, d. Callus induction and roots regeneration in culture medium with release of phenolic compounds, e. Clonal propagation after six months of culture, f. Germplasm conservation after nine months of culture and g. Re-growth after three months in culture medium supplemented with 2.0 mg/L-1 AgNO3.
Table 4 Roots induction from cotyledons and hypocotyls callus of L. huasango, after 45 days of culture
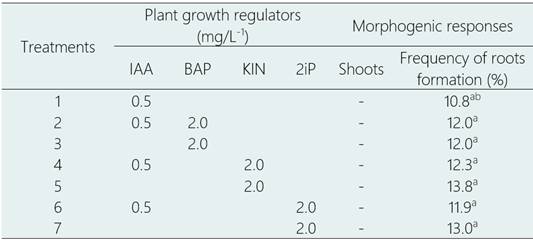
Values within a column followed by a different letter are significantly different at P<0.05 according to Tukey’s multiple range test.
Table 5 Callus and roots induction from cotyledons, hypocotyls and roots explants of L. huasango, after 45 days of culture
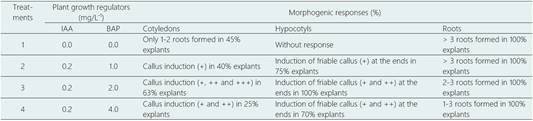
Values within a column followed by a different letter are significantly different at P < 0.05 according to Tukey’s multiple range test.
+, callus covers 1/3 of the explant surface, ++, callus covers ½ of the explants surface and +++, callus completely covers the explants surface.
Clonal propagation
In clonal propagation, induction of shoot elongation from cotyledonary node explants varied slightly with the type and concentration of growth regulator (Table 6). Cotyledonal nodes are widely used explants to initiate a propagation process in vitro, as has been reported in Lupinus albus (Aslam et al., 2020). Shoot elongation was significantly greater in the treatments supplemented with IBA-GA3 and IAA-GA3 with 6.87 and 6.43 cm, respectively, with respect to the treatment supplemented with NAA-GA3 (4.92 cm) (Figure 3e). However, although in the control treatment, without growth regulators, shoot elon gation and nodes formation was significantly greater with 7.63 cm and 13 nodes formed, the physiological state of the in vitro plants, after six months of culture, it was highly detrimental. In all treatments tested, including the control treatment, multiple shoots formation was not observed.
In the additional experiment using treatments with 0.5, 1.0 and 2.0 mg/L-1 BAP, KIN or 2iP the multiple shoots induction was not observed. After three months of culture the shoot apex reached an elongation between 3.5 to 4.5 cm (data not shown in table). In a similar study conducted in plants of SDTF Loja, the multiple shoots induction was not observed in the cotyledonal nodes in the treatments supplemented with 1.0 and 2.0 mg/L-1 BAP, KIN or 2iP or in the treatments supplemented with adenine sulfate 5.0 and 25 mg/L-1 or 20% coconut water (Conde et al., 2017).
The cotyledonary node has previously been used as an explant for multiple shoot induction in Gossypium hirsutum L. (Agrawal et al., 2017), Phaseolus vulgaris L. (Arellano et al., 2009; García et al., 1012), Glossonema varians (Stocks) Benth. ex Hook. (Paliwal et al., 2018), and other species. The cotyledon node meristems, because they have cells competent for regeneration, are tissues widely used in the regeneration of recalcitrant species. (Muruganantham et al., 2007). However, this protocol is only for propagation but not for regeneration plants since axillary meristems are already pre-formed structures and not de novo organized structures (Chero-Ayay et al., 2019). In general, cytokinins play a key role in shoot organogenesis, as has been the case with cotton (G. hirsutum) where among the three cytokinins tested (BA, KIN and TDZ), BA was found to induce more shoots per explant and greater shoot length (Agrawal et al., 2017). However, micropropagation has not only been used to increase the number of propagules but also for the induction and accumulation of secondary metabolites such as phenylpropanoids (Trentini et al., 2021). Similar observations have been reported for Psoralea corylifolia L. for optimization of elicitation condition whit jasmonic acid, characterization, and antimicrobial activity of psoralen from direct regenerated plants (Siva et al., 2015) and shoot regeneration from leaf explants of Whitania coagulans Dunal (Rathore et al., 2016).
Germplasm conservation
Shoot elongation was significantly greater in the treatments supplemented with NAA-GA3 and IBA-GA3 with 8.00 and 7.00 cm, respectively, with respect to the treatment supplemented with IAA-GA3 (6.38 cm), following the same tendency of the results observed in the clonal propagation (Table 7,Figure 3f). Likewise, although in the control treatment, without growth regulators, shoot elongation was significantly greater with 8.45 cm, the physiological state of the in vitro plants, after nine months of culture, it was highly detrimental. In all treatments tested, including the control treatment, multiple shoots formation was not observed. In addition, at all assayed treatments including control was evident a secretion of phenolic compounds in culture medium and the first signs of vitrification for in vitro plants.
Table 6 Effect of PGRs on clonal propagation from seedlings-derived cotyledonal nodes of L. huasango, after six months of culture
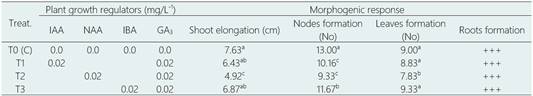
Values within a column followed by a different letter are significantly different at P < 0.05 according to Tukey’s multiple range test.
+++, optimo root system: > roots with more than 5.0 cm in length.
Table 7 Effect of PGRs on germplasm conservation from seedlings-derived cotyledonal nodes of L. huasango, after nine months of culture
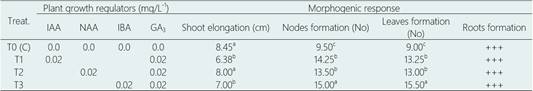
Values within a column followed by a different letter are significantly different at P < 0.05 according to Tukey’s multiple range test.
PGR plant growth regulators, IAA, Indole-3-acetic acid; NAA, α-naphthalene acetic acid; IBA, indole-3-butyric acid; GA3, gibberellic acid. +++, optimo root system: > roots with more than 5.0 cm in length.
Plant biotechnology caused a revolution in the use of plant genetic resources, especially in the conservation of germplasm of clonally propagated species, which are around 1,000 species and around 88,250 species with difficult-to-store seeds (Rajasekharan et al., 2015). In the case of the SDTF species it is possible that most of the species present recalcitrant seeds or with intermediate recalcitrance, reason why it is necessary the in vitro germplasm conservation.
Re-growth
In the re-growth process, from shoot apex and nodal segments obtained from in vitro conserved plants during 9 months of culture, the explants subcultured in the culture medium supplemented with 0.02 mg/L IAA - 0.02 mg/L-1 GA3 not showed growth, and finally died, after three months of culture. This formulation of culture medium was selected because the plants showed an excellent physiological condition, a lower shoot elongation and a high number of formed nodes. Likewise, this formulation has been successfully tested in the in vitro germplasm conservation of numerous Piper species and the numerous species of SDTF Lambayeque (Peru) (Delgado-Paredes et al., 2021). On the other hand, after three months of culture in culture medium supplemented with 2.0 mg/L-1 AgNO3 the survival rates were 30%, the shoot elongation 1.6 cm and without showing signs of vitrification and phenolic secretion (Table 8,Figure 3g).
There is an increasing interest in the use of AgNO3 in the in vitro propagation of cultivated plants, because it is a source of nitrate and favors massive sprouting as well as other morphogenic responses such as organogenesis and somatic embryogenesis. In addition, has also been reported to be a very potent inhibitor of ethylene action and is widely used in plant tissue culture; however, the molecular basis for regulation of morphogenesis under the influence of silver nitrate is completely lacking (Kumar et al., 2009). In Brassica juncea (L.) Coss., AgNO3 in the culture medium, was believed to induce a less-oxidized cellular environment and upregulation of cytokinin biosynthetic genes, which favored shoot multiplication (Paladi et al., 2017), and in white cabbage (Brassica oleracea L.) the utilization of 50 μM AgNO3 promotes the successfully induction and sustaining of regenerative process of anthers cultivated in vitro (Cristea et al., 2012). In Carissa carandas L., an important edible fruit shrub of the Apocynaceae family, a new in vitro propagation protocol was reported, and the compounds quercetin and AgNO3, as inhibitors of auxin transport and the ethylene action in the culture medium, respectively (Bhadane et al., 2018). In another study, microcuttings of passion fruit plants (Passiflora gibertii NEBr.) were placed in MS media with several AgNO3 (0 to 8 mg/L-1) and during one to three months, observing high sensitivity of P. gibertii to ethylene, which led to the loss of vigor and senescence of the explants (Faria et al., 2017). In two potato (Solanum tubersosum L.) cultivars, Granola and Arbolona Negra, did not show epinasty or hyperhidricity symptoms caused by ethylene when were cultivated on MS semi-solid medium supplemented with 2.0 mg/L-1 AgNO3 (Alva & Oropeza, 2013). In Gossypium hirsutum, 2.0 mg/L-1 AgNO3 promotes high-frequency multiple shoot regeneration by inhibiting ethylene production and phenolic secretion, observing the greatest number of shoots per cotyledonary node explant with no phenolic secretion (Prem Kumar et al., 2016).
The explanation of Rathore et al. (2016) on the role of cytokinins in in vitro cultures can be extrapoled to growth regulators in general where differences in explants responses to various plant growth regulators can be explained based on the translocation rates to the responsive regions, their differential uptake, varied effects on metabolic process, and ability to changes the level of endogenous PGRs.
Ex vitro seed germination
After three months of culture only two seeds germinated (4%). This result is correlated with observations in the field where very few individuals were found by natural regeneration.
Table 8 Effect of silver nitrate (AgNO3) on germplasm conservation (re-growth) from apical buds and nodal segments of L. huasango, after three months of culture
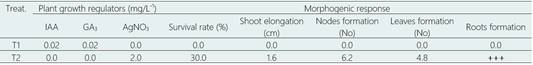
Values within a column followed by a different letter are significantly different at P<0.05 according to Tukey’s multiple range test.
PGR plant growth regulator, IAA, Indole-3-acetic acid; GA3, gibberellic acid; AgNO3, silver nitrate.
+++, optimum root system: > roots with more than 5.0 cm in length.
For this reason, in the establishment of a reforestation program of L. huasango, is recommended in vitro seeds germination, preferably with recently collected seeds. In the study conducted by Conde et al. (2017) in L. huasango of the SDTF of Loja did not report information on ex vitro seeds germination.
4. Conclusions
In this work, some physiological aspects of seed germination, callus induction, clonal propagation, and in vitro germplasm conservation from cotyledonary nodes of L. huasango were studied. In clonal propagation, IBA-GA3 at 0.02 mg/L-1, respectively, produced a higher shoot elongation (6.87 cm/cotyledonary node), however the best physiological condition of the plants was observed in the treatment IAA-GA3 at 0.02 mg/L-1, respectively. Similar results were observed in in vitro germplasm treatments. In the re-growth of apex shoots and nodal segments, the presence of 2.0 mg/L-1 AgNO3 completely arrested phenolic secretion and vitrification from the culture explants. Thus, AgNO3 can be indicated to be a good in vitro clonal propagation and germplasm conservation. The culture medium supplemented with 2,4-D at 0.1 to 5.0 mg/L-1 led the increase of callus induction frequency (60 to 100%) as compared to the control (0%), in several explant types. Root induction was the only morphogenic response observed in cotyledons and hypocotyls callus. Axillary shoot buds of cotyledonary nodes were not activated on several concentrations of cytokinins BAP, KIN or 2iP.
Considering that hualtaco is one of the most threatened species of the seasonally dry tropical forest, this protocol could be used to conserve elite genotypes, clonal micropropagation and the establishment of a refores-tation program with in vitro seeds germination. Likewise, in the rapid roots induction and the establishment of cell suspensions to produce economically important secondary metabolites.