1. Introduction
Orchidaceae family encompasses hundreds of species, which are considered the most evolutionarily developed in the plant kingdom (Lu et al., 2019). For years, they have been used in different areas. Since, its research and medical purposes (Bhattacharyya & Van Staden, 2016; Pant et al., 2021), as food (Calva et al., 2018), and ornamental (Hinsley et al., 2018; Li et al., 2021). The latter due to their extravagant colours and shapes, which makes them peculiar and favourite for decoration. In this group of ornamentals, Cattleya and Phalaenopsis are among the most commercial (Hinsley et al., 2018).
Like other plants, orchids need to be under the right conditions of temperature (about 26°C), not be exposed to direct sunlight, with a photoperiod of 16 light hours, and light soil (Wang et al., 2019; Chandra, 2020).
An uncontrolled extraction has caused certain species to become endangered (Fay, 2018; Williams et al., 2018; Gale et al., 2019). In addition, orchids have a long production period, and, for this reason, other methods are applied for its development and conservation.
In vitro culture or micropropagation is the most used alternative for orchids propagation (Freitas et al., 2021), being applied in the stages of germination, seedling growth and rooting; especially for commercial species (Arellano et al., 2020; Cardoso et al., 2020). For this purpose, culture media are used, sometimes complemented with organic substances like banana flour, coconut water and others (Salazar & Botello, 2020; Wida & Hariyanto, 2020) or growth regulators (Parthibhan et al., 2015), to provide the physiological stimulus necessary for their development. Moreover, this method allows to provide the right temperature and light conditions for the development of plants (Calevo et al., 2020; Kang et al., 2020). These conditions reduce the development period of plants, facilitating their massive production.
Therefore, micropropagation is a technique that helps the production of orchids. Although it has been improved and implemented over the years, it still needs to be perfected, in order to generate fewer losses, either as a commercial product or for conservation purposes, especially considering that there are numerous species, and each one reacts differently any given growing medium. For this reason, this study aimed to determine the optimal propagation conditions for the species Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume, considering multiplication and rooting phase. For this purpose, growth regulators were used: cytokinins for multiplications, and auxins for rooting phase.
2. Materials and methods
Study site and plant material
This investigation consisted in two experiments: multiplication and rooting, which were developed at tissue culture laboratory of Biotechnology Institute (BTI), Universidad Nacional Agraria La Molina, Lima, Perú. In both multiplication and rooting, Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume were used.
Multiplication experiments were conducted using C. maxima and P. amabilis seedling with one or two leaves, while, rooting experiment were conducted using seedling with three or four leaves. This material was obtained by sowing in culture medium of seeds extracted from C. maxima and P. amabilis capsules, which were developing for a year, under 24 °C and 16 h photoperiod.
Treatments
Firstly, Murashige and Skoog (MS) medium were prepared with distilled water, and supplemented with micro elements, macro elements, sucrose, and vitamins. This was the basal culture medium for both experiments. Depending on the experiment and treatment, MS medium was supplemented with cytokinins (kinetin and 6-Benzylaminopurine), auxins (Indol-3-butiric acid, Naphthaleneacetic acid or 2,4-Dichlorophenoxyacceit acid) or natural additives (green banana flour, with high content of carbohydrates, in addition to: potassium, protein, iron, calcium, and phosphorus). The culture medium pH was adjusted to 5.5, in case the value increased or decreased, HCl or NaOH was added, respectively. Agar (4 g.L-1) was incorporated, except when banana flour was added, in that case agar was not used. Culture medium was poured into a jar, previously disinfected and autoclaving at 121°C for 20 minutes.
Table 1 Multiplication experiment treatments in Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume
| Basal medium | Complement concentration | Species |
| MS + 4 g.L-1 agar | - | C. maxima |
| MS | 50 g.L-1 banana flour | C. maxima |
| MS + 4 g.L-1 agar | 0.003 g.L-1 kinetin | C. maxima |
| MS + 4 g.L-1 agar | 0.005 g.L-1 BAP | C. maxima |
| MS + 4 g.L-1 agar | - | P. amabilis |
| MS | 50 g.L-1 banana flour | P. amabilis |
| MS + 4 g.L-1 agar | 0.003 g.L-1 kinetin | P. amabilis |
| MS + 4 g.L-1 agar | 0.005 g.L-1 BAP | P. amabilis |
MS: Murashige and Skoog, BAP: 6-Benzylaminopurine.
Multiplication experiment was formed by the application of MS + 4 g.L-1 agar; MS + 50 g.L-1 banana flour; MS + 4 g.L-1 agar + 0.003 g.L-1 kinetin and MS + 4 g.L-1 agar + 0.005 g.L-1 6-Benzylaminopurine (BAP) on Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume given as a result eight treatments (Table 1). Consequently, each treatment had 40 repetitions.
Conversely, the rooting experiment tested the effect of five different media: (i) MS + 4 g.L-1 agar; (ii) MS + 50 g.L-1 banana flour; (iii) MS + 4 g.L-1 agar + 0.005 g.L-1 Indol-3-butiric acid (IBA); (iv) MS + 4 g.L-1 agar + 0.003 g.L-1 Naphthalene acetic acid (NAA) and (v) MS + 4 g.L-1 agar + 0.003 g.L-1 2,4 Dichlorophenoxyacetic acid (2,4-D) on Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume given as a result ten treatments (Table 2), with a total of 36 repetitions for each.
Table 2 Rooting experiment treatments in Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume
| Basal medium | Complement concentration | Species |
| MS + 4 g.L-1 agar | - | C. maxima |
| MS | 50 g.L-1 banana flour | C. maxima |
| MS + 4 g.L-1 agar | 0.005 g.L-1 IBA | C. maxima |
| MS + 4 g.L-1 agar | 0.003 g.L-1 NAA | C. maxima |
| MS + 4 g.L-1 agar | 0.003 g.L-1 2,4-D | C. maxima |
| MS + 4 g.L-1 agar | - | P. amabilis |
| MS | 50 g.L-1 banana flour | P. amabilis |
| MS + 4 g.L-1 agar | 0.005 g.L-1 IBA | P. amabilis |
| MS + 4 g.L-1 agar | 0.003 g.L-1 NAA | P. amabilis |
| MS + 4 g.L-1 agar | 0.003 g.L-1 2,4-D | P. amabilis |
MS: Murashige and Skoog, IBA: Indol-3-butiric acid, NAA: Naphthalene acetic acid, 2,4 D: 2,4 Dichloro-phenoxyacetic acid.
Evaluation variables
For the multiplication experiment the shoot length (mm) was measured, and the number of leaves and shoots was quantified, while, for rooting experiment, shoot length (mm), number of leaves, and presence of roots (%) were recorded. In both, multiplication and rooting experiment the sampling dates were at 30, 45, 60, 75 and 90 days after sowing.
Statistical analysis
Both experiments were arranged in completely randomized design. The data obtained was submitted to ANOVA and Scott-Knot test (95%) using the statistical software AGROESTAT (Barbosa & Maldonado, 2015).
3. Results and discussion
Shoot length of Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume in multiplication and rooting experiment
As mentioned, the present research was composed of two experiments: multiplication and rooting of C. maxima and P. amabilis. The first variable measured was shoot growth (Figure 1). In Table 3 (multiplication) and Table 4 (rooting), shoot length of all treatments increased in value over time. As well, the treatments on C. maxima presented shoot length higher than those on P. amabilis (P ≤ 0.05). This result may be explained by genetic different among those species.
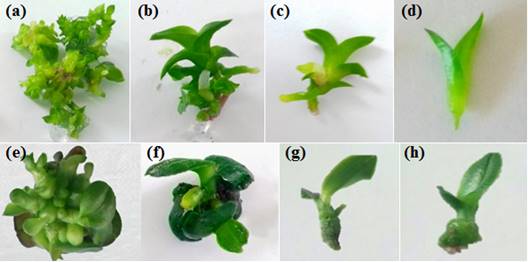
Figure 1 Effect of culture medium on Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume. multiplication at 90 days after sowing. (a) Effect of 6-Benzylaminopurine (BAP) on C. maxima for number of shoots, (b) Effect of banana flour on C. maxima for number of shoots, (c) Effect of 6-Benzylaminopurine (BAP) on C. maxima for shoot length, (d) Effect of banana flour on C. maxima for shoot length, (e) Effect of 6-Benzylaminopurine (BAP) on P. amabilis for number of shoots, (f) Effect of kinetin on P. amabilis number of shoots, (g) Effect of 6-Benzylaminopurine (BAP) on P. amabilis for shoot length, (h) Effect of banana flour on P. amabilis for shoot length.

Figure 2 Effect of culture medium on the shoot length of Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume in the rooting experiment at 90 days after sowing. (a) Effect of banana flour on C. maxima, (b) Effect of Indol-3 butiric acid (IBA) on C. maxima, (c) Effect of Naphthalene acetic acid (NAA) on P. amabilis, (d) Effect of Indol-3-butiric acid (IBA) on P. amabilis.
Murashige and Skoog (MS) is a culture medium often used in the micropropagation technique, and it is composed by macro and micronutrients, vitamins, and hormones (Ugale & Barwant, 2020), which provide optimal conditions to aerial and root growth of explants. However, the presence of other substances may enhance or limit their effect. C. maxima in the medium MS + banana flour was the highest at 90 DAS (Days After Sowing); however, the same treatment significantly limited the growth of P. amabilis (P ≤ 0.05). Inside the group of C. maxima, MS + banana flour displayed a remarkable effect which initially showed a value of 3.25 mm at 30 DAS and in late reached a value of 11.5 mm at 90 DAS meaning an increment of 254%. Likewise, MS + agar + kinetin was the most prominent within the group of P. amabilis presenting shoot length of 2.35 mm at 30 DAS, and 8.98 mm at 90 DAS (Table 3).
In the Table 4 (rooting), within the group of C. maxima, MS + agar + NAA increased considerably the height of seedlings until 12.4 mm at 90 DAS. Nevertheless, MS + banana flour highlighted among the treatments on P. amabilis (P ≤ 0.05) (Figure 2). It was also the most prominent of all culture medium used for this variable. We can infer that differentiated responses within each group of C. maxima and P. amabilis plants demonstrated that the genetic factor is key when we have to select and adapt a suitable culture medium.
The positive effect of banana flour may be related to its more complete composition than the application of hormones such as kinetin and 6-Benzylaminopurine (BAP), or Indol butyric acid (IBA), Naphthalene acetic acid (NAA) and 2,4 D (Table 3and4). It has been reported that banana flour can content macro nutrient (Na, K, Ca, Mg, Fe and P) (Munyawera, 2016), sugars (starch and amylose) and protein (Da Mota et al., 2000), enriching entirely the culture medium that gave rise to highest plants. In addition, the use of cytokinins as kinetin and BAP, in micropropagation, is more related to formation of shoots than increase of plantlet height (Podwyszynska, 2003; Ashraf et al., 2014). In this experiment, kinetin and BAP had the same effect on shoot elongation (Table 3). Some authors such as Munyawera (2016) have even claimed that banana flour may replace to MS. Vilcherrez et al. (2020) also found positive effect of banana flour on in vitro propagation of C. maxima.
It can also be seen in Table 4 that IBA, NAA and 2,4 D had differenced effect on stem elongation of C. maxima and P. amabilis (P ≤ 0.05), in fact, while NAA stimulated the plantlet height, 2,4 D limited drastically it, suggesting that the latter had phytotoxic effect as mentioned to another crop as Allium cepa, where Özkul et al. (2016) reported that 2,4 D modified negatively the mitotic phases given as a result the diminishing of Mitotic Index. Similar behaviour of different auxins was observed in other experiment with Elaeis guinenesis, where not all auxins used brought about the same result (Jayanthi et al., 2015).
It can also be seen in Table 4 that IBA, NAA and 2,4 D had differenced effect on stem elongation of C. maxima and P. amabilis (P ≤ 0.05), in fact, while NAA stimulated the plantlet height, 2,4 D limited drastically it, suggesting that the latter had phytotoxic effect as mentioned to another crop as Allium cepa, where Özkul et al. (2016) reported that 2,4 D modified negatively the mitotic phases given as a result the diminishing of Mitotic Index. Similar behaviour of different auxins was observed in other experiment with Elaeis guinenesis, where not all auxins used brought about the same result (Jayanthi et al., 2015).
Number of shoots (in multiplication experiment) and leaves (in multiplication and rooting experiment) of Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume
In Table 5 (Figure 1) (multiplication experiment), the culture medium had a differential effect on number of shoots in both species, C. maxima and P. amabilis. Actually, the treatments on C. maxima increased significantly its number of shoots when compared to plants of P. amabilis (P ≤ 0.05). The number of shoots of the former ranged from 3 to over 22; while the latter had between 2 and 6 shoots. In general, MS + agar + BAP on C. maxima raised the number of shoots reaching to almost 23; while, MS + banana flour and MS + agar + Kinetin decreased this variable until reaching almost 3 shoots (P ≤ 0.05) on P. amabilis.
Regarding number of leaves (Table 6) (multiplication experiment), the plants of the group of C. maxima presented more leaves than P. amabilis . The former showed a number of leaves which ranged from 7 to almost 10 at 90 DAS, while the latter displayed between 2 and almost 5 leaves (Table 6). Likewise, the most outstanding were MS + agar + Kinetin and MS + agar + BAP by 9.71 and 9.75 leaves, respectively in C. maxima. On the contrary, those same treatments decreased importantly the number of leaves in P. amabilis reaching values of 2.14 and 3 leaves.
In rooting experiment, we also evaluated the number of leaves (Table 7). It was observed that MS+ banana flour, MS + agar + IBA and MS + agar + NAA improved this variable. In fact, they raised the number of leaves of C. maxima up to 10.1, 17.7 and 21.7 at 90 DAS respectively, being MS + agar + IBA and MS + agar + NAA the most remarkable. Interestingly, all plants of P. amabilis had fewer leaves than C. maxima at the end of the experiment (P ≤ 0.05).
Table 3 Effect of different culture medium on the shoot length (mm) in Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume in the multiplication experiment
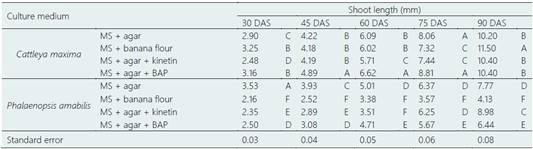
DAS: days after sowing; MS: Murashige and Skoog; Agar: 4 g.L-1; Banana flour: 50 g.L-1; Kinetin: 0.005 g.L-1; BAP (6-Benzylaminopurine): 0.005 g.L-1. Different letters indicate statistical difference (P ≤ 0.05) inside each column.
Table 4 Effect of different culture medium on shoot length in Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume in the multiplication experiment
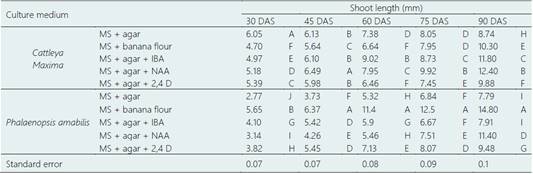
DAS: days after sowing. MS: Murashige and Skoog. Agar: 4 g.L-1. IBA (Indol-3-butiric acid): 0.005 g.L-1. NAA (Naphthalene acetic acid): 0.003 g.L-1. 2,4 D (2,4 Dichlorophenoxyacetic acid): 0.003 g.L-1. Different letters indicate statistical difference (P ≤ 0.05) in each column.

Figure 3 Effect of different culture medium in Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume rooting for presence of roots. DAS: days after sowing, MS: Murashige and Skoog, IBA: Indol-3-butiric acid, ANA: Naphthalene acetic acid, 2,4 D: 2,4 Dichlorophenoxyacetic acid.
Table 5 Effect of different culture medium on the number of shoots in Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume in the multiplication experiment

DAS: days after sowing; MS: Murashige and Skoog; Agar: 4 g.L-1; Banana flour: 50 g.L-1; Kinetin: 0.005 g.L-1; BAP (6-Benzylaminopurine): 0.005 g.L-1. Different letters indicate statistical difference (P ≤ 0.05) inside each column.
Table 6 Effect of different culture medium on the number of leaves in Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume in the multiplication experiment

DAS: days after sowing; MS: Murashige and Skoog; Agar: 4 g.L-1; Banana flour: 50 g.L-1; Kinetin: 0.005 g.L-1; BAP (6-Benzylaminopurine): 0.005 g.L-1. Different letters indicate statistical difference (P ≤ 0.05) inside each column.
Table 7 Effect of different culture medium on number of leaves in Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume in the rooting experiment
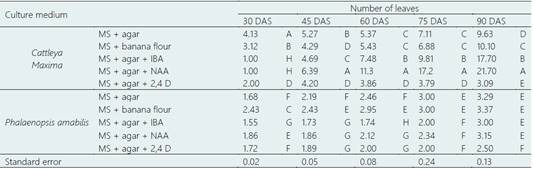
DAS: days after sowing. MS: Murashige and Skoog. Agar: 4 g.L-1. IBA (Indol-3-butiric acid): 0.005 g.L-1. NAA (Naphthalene acetic acid): 0.003 g.L-1. 2,4 D (2,4 Dichlorophenoxyacetic acid): 0.003 g.L-1. Different letters indicate statistical difference (P ≤ 0.05) in each column.
As mentioned, kinetin and BAP, IBA and NAA increased the number of shoots and leaves in multiplication and rooting experiment (P ≤ 0.05) (Table 5,6 and7), respectively in C. maxima. The results confirm other ones that show high efficacy in the use of cytokinins to increase the number of shoots (Podwyszynska, 2003; Weiser et al., 2020). In addition, in this investigation the effectiveness of BAP was more notable than IBA (P ≤ 0.05). Although, other researchers, in Cucumis sativus, found that kinetin is more recommendable than BAP (Abu-Romman et al., 2015), suggesting more investigation in this field of study.
Percentage of roots of Cattleya maxima Lindl. and Phalaenopsis amabilis (L.) Blume in the rooting experiment
The percentage of roots was evaluated as well (Figure 3). IBA, NAA and 2,4-D had modulator effect by inhibiting the root formation in C. maxima. On contrary, banana flour stimulated considerably the growth of this organ in C. maxima, in the rooting experiment (P ≤ 0.05). On the other hand, the action of banana flour, IBA and NAA were positive in term of increasing the percentage of root in P. amabilis. According to Borjas et al. (2020), one of main uses of auxins is to promote the rooting in micropropagation, especially in low concentration, for example in E. guineensis, Gomes et al. (2015) displayed almost 54 µM of IBA promoted the presence of rooted seedlings and the number of roots.
In the case of 2,4-D, it was evidenced that this molecule had phytotoxic effect, because it completely limits root growth. Indicating likely that the dosage used in this experiment was higher. According to Song (2014), in low concentration, 2,4 D can replace of IAA (Indol 3-acetic acid), stimulate plants defence, and induce various hormones such as salicylic and jasmonic acid.
4. Conclusions
Cattleya maxima showed better results than Phalaenopsis amabilis, since in all the variables studied, the former presented the highest values. Moreover, each complement added to the MS, affected the evaluated characteristics differently. Banana flour (50 g.L-1) increased the height of C. maxima seedling in the multiplication experiment, and of P. amabilis seedlings in the rooting experiment. In the C. maxima multiplication experiment, when the medium was supplemented by kinetin (0.003 g.L-1) or BAP (0.005 g.L-1), the number of shoots and leaves increased. While, in the rooting experiment, NAA (0.003 g.L-1) and IBA (0.005 g.L-1) significantly increased the number of leaves in C. maxima. The formation of roots in C. maxima was stimulated by banana flour (50 g.L-1), while in P. amabilis, the greatest stimulus was given by NAA (0.003 g.L-1) and IBA (0.005 g.L-1). Finally, It was observed that 2,4-D (0.003 g.L-1) cause phytotoxic effects and inhibit root formation.
Future research should focus on adapting this technique for use in decreasing overharvesting and promoting conservation of these species.















