1. Introduction
Maize, Zea mays L. (Poaceae), is a crop widely sown in the world since it can adapt to various agroecological conditions (Suganya et al., 2020). In Ecuador, 1,572,943 tons of maize are produced annually, from 328,214 ha harvested, and it is the second most important crop after cacao (MAG, 2020). Throughout its phenological cycle, maize can be attacked by approximately 250 species of insects and mites worldwide (Singh & Singh, 2018), of which in Ecuador the following are considered of economic importance, the fall armyworm, Spodoptera spp., the corn earworm, Heliothis zea (Boddie, 1850) (Lepidoptera: Noctuidae), the stem borer, Diatraea spp. (Lepidoptera: Crambidae), thrips (Frankliniella spp.) (Thysanoptera: Thripidae), the leafhopper, Dalbulus maidis (DeLong, 1923) (Hemiptera: Cicadellidae) and the corn leaf aphid, Rhopalosiphum maidis (Fitch, 1856) (Hemiptera: Aphididae) that can severely limit production (Caviedes et al., 2022; Quito-Avila et al., 2016).
The corn leaf aphid, R. maidis stands out as a pest because it can cause damage in the different growth stages of corn, but mainly in the vegetative phase, in which the nymphs and adults feed on all parts of the plant. In the reproductive phase, this aphid interferes with pollination and introduces fungi to the cob (Alam et al., 2020). In addition, to the direct damage caused by sucking photoassimilates from the plant, the aphid is also a vector of some viruses that negatively impact production, namely, Maize yellow dwarf virus (MDMV), Barley yellow dwarf virus (BYDV), Sugarcane mosaic virus (SCMV), and Cucumber mosaic virus (CMV) (Chen et al., 2019; Quito-Avila et al., 2016).
As a fundamental basis for the management of phytosanitary problems of maize, it is necessary to review its ecological bases as an agroecosystem. Consumers turn to plant crops (first trophic level) as synthesizers of organic matter to obtain vital resources that they cannot synthesize, generating consumption chains. The second trophic level is made up of herbivores or phytophagous organisms (some with pest status), the third by primary carnivores (parasitoids and predators), which are interconnected by sharing functions in one of their links (e.g., a predator consuming in two or more food chains), thus forming complex networks, structurally and functionally. Additionally, hyperparasitism is involved in the fourth trophic level as a highly evolved relationship that exists between entomophages (Kos et al., 2012).
Aphids constitute one of the most important groups of pests in agriculture, causing economic damage to many crops worldwide (Van Emden & Harrington, 2007). Aphidophagous braconids (Hymenoptera: Braconidae) play an important role in population regulation and can attack more than one aphid-host species (Kos et al., 2012). On the other hand, hyperparasitoids have a great impact on the dynamics of arthropod communities, and their effect on biological control programs remains uncertain (Kos et al., 2012; Sullivan & Völkl, 1999). Likewise, predators represent natural biological control agents of aphids, among them hoverflies (Diptera: Syrphidae) and coccinellids (Coleoptera: Coccinellidae) (Amorós-Jiménez & Marcos-García, 2020; Bouvet et al., 2021). Despite the importance of R. maidis as a corn pest in Ecuador, little is known about the multitrophic associations that are entangled around this pest. Given the relevance of natural enemies in the design of integrated pest management (IPM) strategies, as well as for the establishment of applied biological control programs, this study aimed to determine the insect species that make up the food webs associated with the corn leaf aphid, R. maidis in a cornfield in the province of Manabí, Ecuador.
2. Materials and methods
This study was carried out during the year 2021 in the town of Charapotó, Manabí province, Ecuador (00°49′15″S, 80°30′18″W, 30 m a.s.l., Figure 1). The climatic conditions correspond to a tropical dry forest: average annual temperature: 25 °C (range: 24.74 °C to 30.9 °C), relative humidity: 72%, average annual rainfall: 550 mm per year (INAMHI, 2021). A 1000 m² corn plot of the INIAP 583® variety was established, planted at a distance of 0.20 m between plants and 0.80 m between rows. Within the lot, 60 rows which were not treated with chemical insecticides were sampled weekly, from which 36 leaves on 18 plants were randomly selected and taken to the Entomology Laboratory, Faculty of Agricultural Engineering, Technical University of Manabí for examination.
The samples were observed under a Carl-Zeiss® stereo scope (magnification: 18-40X) and the numbers of live and parasitized aphids were counted. Parasitized aphids were recognized by their inflated integument and brown coloration (mummies). Mummies were placed individually in transparent gelatin capsules to the emergence of adult parasitoids and allow later identification.
Larvae of hoverflies (Diptera: Syrphidae) preying on R. maidis were observed on the leaves and were reared to adults for identification. Small wasps emerged from hoverfly pupae, which were identified using dichotomous keys of Narendran et al. (2007) and Xiao et al. (2009).
Individuals of lady beetles (Coleoptera: Coccinellidae) preying on R. maidis were observed in the field, and were collected into flasks containing 70% ethyl alcohol and the species were identified by comparison with voucher specimens collected in previous studies carried out in the same province (Bailon et al., 2022).
Hoverfly adults were identified with the keys of Marinoni et al. (2007) and Mengual et al. (2018). The hymenopteran parasitoids emerged from the parasitized aphids were identified using the key provided by Zamora-Mejías and Hanson (2017). Trophic interactions between detected taxa were also plotted. All identifications were made in the Entomology Laboratory, Faculty of Agricultural Engineering, Technical University of Manabí, where 180 voucher specimens (phytophagous and natural enemies) were deposited.
3. Results and discussion
Trophic networks
Nine taxa of natural enemies were detected associated with R. maidis and their interconnections are shown in Figure 2. From the 775 parasitized aphids emerged the primary parasitoid, Lysiphlebus testaceipes Cresson, 1880 (Hymenoptera: Braconidae) and the hyperparasitoid, Syrphophagus aphidivorus (Mayr, 1876) (Hymenoptera: Encyrtidae) (Figure 3).
The larvae of a hoverfly species preying on R. maidis was identified as Ocyptamus dimidiatus (Fabricius, 1781) (Diptera: Syrphidae) and the parasitic wasps that emerged from the pupae of O. dimidiatus were identified as Pachyneuron formosum Walker, 1833 (Hymenoptera: Pteromalidae) (Figure 3). An undetermined species of assassin bug, Zelus sp. (Hemiptera: Reduviidae) was found preying on R. maidis. Furthermore, the following lady beetles (Coleoptera: Coccinellidae) were also found feeding on R. maidis: Cheilomenes sexmaculata (Fabricius, 1781), Cycloneda sanguinea (Linnaeus, 1763), Hippodamia convergens Guerin-Meneville, 1842, and Paraneda pallidula guticollis (Mulsant, 1850). Four trophic levels around R. maidis are entangled (Figure 2), with maize as the host plant at the first trophic level and the pest at the second trophic level. The third trophic level showed the highest number of taxa, one primary parasitoid and six predators. Interestingly, the assassin bug, Zelus sp., in addition to preying on R. maidis attacked also coccinellids, thus consuming at two levels. Finally, in the fourth trophic level, hyperparasitoids associated with the primary parasitoid of R. maidis, as well as the parasitoid of the predatory hoverfly were detected.
Species detected for the first time in Ecuador
Syrphophagus aphidivorus (Mayr, 1876) (Hymenoptera: Encyrtidae) (Adapted from Iemma et al. (2017) and Zamora-Mejías & Hanson (2017)).
Diagnosis. Female: Length 1.0 ‒ 1.5 mm, bluish-green, bronze body (Figure 3a). Geniculate antennae with the scape longer than other segments (Figure 3b). Forewing with small pterostigma, very short marginal vein and dark brown venation (Figure 3c), axillae almost touching in the middle region of the mesoscutellum. Median tibia with a large spur at the apex (Figure 3d).
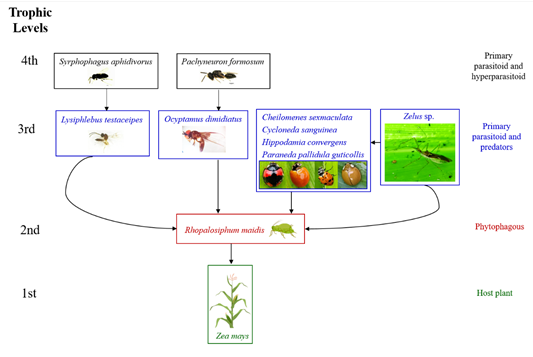
Figure 2 Natural enemies associated with Rhopalosiphum maidis on maize. The black arrows show the interconnections in the agroecosystem. Herbivore (aphid pest) in red, primary carnivores in blue and secondary carnivores in black. The pictures of maize plant and aphid were created in Biorender.
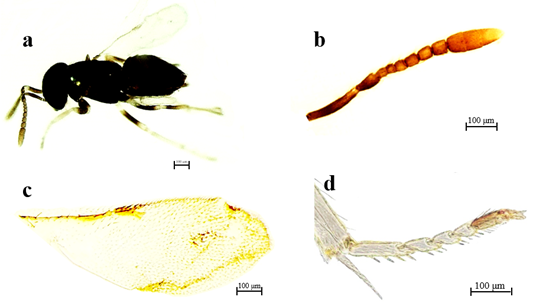
Figure 3 Syrphophagus aphidivorus (Mayr, 1876): (a) Habitus of female, lateral view; (b) Antenna; (c) Wing; (d) Mesothoracic tibia showing a long thick spur.
Pachyneuron formosum Walker, 1833 (Hymenoptera: Pteromalidae) (Adapted from Xiao et al. (2009)).
Diagnosis. Female: Length ca. 2.0 mm, body green with metallic reflections; antenna brown except scape and pedicel yellowish brown; leg yellow or brown except coxa which is similar to body color; gaster brown (Figure 4a and 4b). Antennal formula 1-1-2-6-3 (Figure 4c); scape reaching anterior ocellus; funiculus segments 1.5X as long as wide (Figure 4c). Head broader than thorax dorsally (Figure 4b). Marginal vein 4X times longer than maximum width, marginal vein longer than stigma (1.2X). Gaster wider than thorax, 1.2X as long as wide, with reticulate petiole longer than wide.
The natural enemies observed constitute important biological control agents associated with the corn leaf aphid. Lysiphlebus testaceipes belongs to the subfamily Aphidiinae (Hymenoptera: Braconidae) that includes solitary endoparasitoids, koinobionts, exclusive to adult and immature aphids (Zamora-Mejías & Hanson 2017). This parasitoid in Ecuador had not been reported associated with R. maidis in maize. In fact, hitherto, L. testaceipes had been reported in Ecuador as a parasitoid associated with the species Aphis gossypii Glover, 1877 and Myzus persicae (Sulzer, 1776) (Hemiptera: Aphididae) in cotton, melon, pepper and tobacco crops (Chirinos et al., 2021).
From a high percentage (38.2%) of the reared aphid mummies, the hyperparasitoid S. aphidivorus emerged, which translates to an adult emerging from each mummy. This is the first record of the species for Ecuador. Iemma et al. (2017) reported that S. aphidivorus is a secondary endoparasitoid of other hymenopteran parasitoids, in which hyperparasitoid females have two oviposition strategies: ovipositing in live parasitized aphids, as well as in mummified aphids. Both strategies aim to attack the larvae of primary parasitoids (Iemma et al., 2017).
The species L. testaceipes and S. aphidivorus have been found in other field studies in different geographical regions. Studies carried out by Villa-Ayala et al. (2020) detected L. testaceipes as a primary parasitoid of the yellow aphid, Melanaphis sacchari (Zehntner, 1897), on sorghum crop in the state of Morelos, Mexico, and among the hyperparasitoids they observed S. aphidivorus. Research carried out by Iemma et al. (2017) on alfalfa crop in the state of São Paulo, Brazil, reported S. aphidivorus, as a secondary parasitoid of Aphidius ervi (Haliday, 1834) (Hymenoptera: Braconidae). Zamora-Mejías & Hanson (2017) sampled plants infested with aphids in various regions of Costa Rica and detected nine species of primary parasitoids, including L. testaceipes and hyperparasitoids, including S. aphidivorus.
The species of predatory coccinellids and syrphids found in this study had been previously reported for Ecuador. Bailon et al. (2022) in an inventory carried out in three geographical areas of the province of Manabí detected 13 taxa of coccinellids associated with aphids in corn, with Ch. sexmaculata being the most abundant species followed by C. sanguinea and H. convergens. The hoverfly, O. dimidiatus was listed by Marín-Armijos et al. (2017) as part of the syrphid fauna of Ecuador. On the other hand, Fortoul-Diaz et al. (2020) carried out a field study on sorghum in the state of Puebla, Mexico and identified among the predators associated with M. sacchari, the coccinellids H. convergens and C. sanguinea, followed in abundance by various species the hoverflies. Maza et al. (2022) recorded for the first time three species of hoverflies, O. dimidiatus, Ocyptamus gastrostactus (Wiedemann, 1830) and Toxomerus watsoni (Curran, 1930), whose larvae were found feeding on whiteflies and aphids on pepper in Tucumán, Argentina. Fidelis et al. (2018) found that the mortality of M. persicae was a consequence of the predation exerted by larvae of various species of coccinelids, including C. sanguinea, together with the action of hoverflies species.
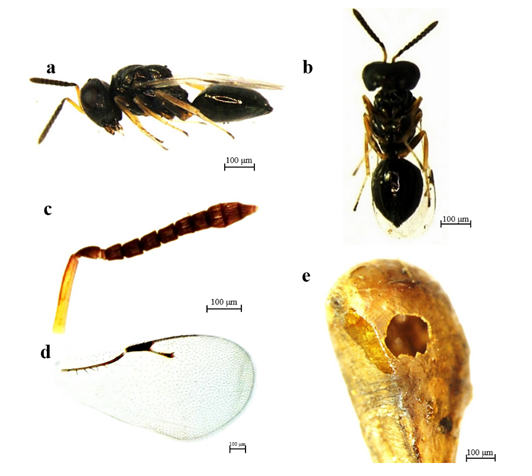
Figure 4 Pachyneuron formosum Walker, 1833: a and b, Habitus of female, lateral and ventral view; c, Antenna; d, Wing; e, Pupa of Ocyptamus dimidiatus (Fabricius, 1781) showing exit hole perforated by P. formosum.
In our study, P. formosum was detected for the first-time parasitizing O. dimidiatus pupae, with approximately five adults emerging from each pupa. Pachyneuron is a cosmopolitan genus as they can be found as hyperparasitoids of Hemiptera (Aphidoidea, Coccoidea and Psylloidea) through Hymenoptera (Ichneumonoidea: Braconidae, Aphidiinae; Chalcidoidea: Encyrtidae, Aphelinidae), or primary and secondary parasitoids of predators of various orders, i.e., Diptera, Coleoptera (Coccinellidae), and Neuroptera (Chrysopidae) (Dzhanokmen, 2009). Previous studies have reported P. formosum as a parasitoid of hoverflies in other regions. In Turkey, Tek & Okyar (2018) found individuals of P. formosum and individuals of Euneura lachni (Ashmead, 1887) (Hymenoptera: Pteromalidae), emerging together from an unidentified hoverfly puparium. Santo et al. (2018) observed and identified parasitized pupae of two syrphid species Episyrphus balteatus (De Geer, 1776) and Sphaerophoria scripta (Linnaeus, 1758), from which hymenopterans of the Ichneumonidae (Diplazontinae) and Pteromalidae families emerged.
The combined action of parasitoids and predators could contribute to the reduction of population densities of R. maidis in maize. The biological control of aphids is exerted by numerous parasitoids belonging to the families Braconidae, Encyrtidae and other Chalcidoidea (Bañol et al., 2017; Zamora-Mejías & Hanson, 2017) as well as predators of the families Coccinellidae and Syrphidae are present in most ecosystems, both agricultural and urban (Amorós-Jiménez & Marcos-García, 2020; Fortoul-Diaz et al., 2020). Hyperparasitoids have been pointed out for causing adverse effects in classical biological control programs, but hyperparasitoids cannot be eliminated from food webs (Tougeron & Tena, 2019). Consequently, studies on their ecology in different environmental conditions are necessary to estimate the interactions between species and their function in trophic networks (Tougeron & Tena, 2019). This research constitutes a significant contribution to the knowledge of the occurrence of natural enemies in an ecological system surrounding mayze crop. The information on the trophic associations around R. maidis will contribute to the planning of IPM strategies.
4. Conclusions
Nine taxa of natural enemies associated with R. maidis were found in this study, and we record for the first time for Ecuador the species S. aphidivorus (Hymenoptera: Encyrtidae) attacking L. testaceipes (Hymenoptera: Braconidae) and P. formosum (Hymenoptera: Pteromalidae) parasitizing pupae of the aphid-feeding hoverfly, O. dimidiatus (Diptera: Syrphidae). In terms of biological control, these results increase the knowledge of four-trophic interactions between maize plants, aphids, aphidophagous parasitoids, hyperparasitoids, predators consuming in more than one network, and predators and parasitoids interacting within an agroecosystem.
Future studies should be conducted to elucidate how tertiary consumers (fourth trophic level), i.e., hyperparasitoids and predators (e.g., Zelus sp.), affect the efficacy of secondary consumers (third trophic level), i.e., primary parasitoids and predators.