1. Introduction
Listeria monocytogenes is an important bacterial species belonging to the genus Listeria in the Listeriaceae family (Aktop et al., 2020). It is a pathogenic microorganism widely found in food and food processing environments and in nature. Listeria is a dangerous bacteria group in the food production and consumption field because of its ability to grow at low temperatures and maintain survival for a long period of time in the food processing environment (McLauchlin et al., 2014). The sources of contamination of L. monocytogenes include seafood, poultry, milk and milk products, meat and meat products, vegetables, fresh fruits, fruit juices, and ready-to-eat foods in particular (Iannetti et al., 2020; Sibanda & Buys, 2022).
L. monocytogenes is the main causative agent of listeriosis in humans and animals (Yavuz & Korukluoğlu, 2010; Magalhães et al., 2014; Ramos et al., 2014). Even though this disease is mild with symptoms of fever, muscle pain, nausea, and diarrhea, it causes complications in pregnant women and significant health problems in newborns, people over 65 years of age, and immunocompromised people (Osek et al., 2022). It is also a threat factor to public health as it has a high mortality rate (20% - 30%) along with other diseases such as meningitis, septicemia, endocardi tis, and gastroenteritis (Orsi & Wiedmann, 2016; Matthews et al., 2017; Budiati et al., 2020; Iannetti et al., 2020). The contamination of food with >100 CFU/g dose of L. monocytogenes during food production, distribution, and storage and the consumption of this contaminated food are the main reasons for listeriosis; hence, Listeria has been accepted as a food-borne pathogen (Farber et al., 2021).
L. monocytogenes is known to be a resistant bacterial species as it can survive even under adverse environmental conditions through its different physiological and ecological properties (Osek et al., 2022). Nevertheless, there are different methods to control the growth of this bacterium and prevent food-borne epidemics, including cooling, freezing, lowering water activity, acidification, modified atmosphere storage, and use of antimicrobial agents such as bacteriocin and species-specific bacteriophage (Şanlıbaba & Uymaz, 2015; Altuntaş & Korukluğlu, 2018). The direct application of chemical agents to fresh fruits, vegetables, and ready-to-eat foods to reduce contamination of pathogens in the food industry is inconvenient (Şanlıbaba & Uymaz, 2015). Therefore, innovative control strategies are being increasingly sought to reduce the microbial load of raw materials; control the potential risk of pathogens in the food chain, including processing and storage; and meet consumer requirements for minimally processed food with minimal protective additives. Regarding the application of biocontrol treatments as an alternative to chemical preservatives, there has been increasing interest in the use of natural antimicrobial agents, including vinegar (acetic acid), lemon juice (citric acid), carbonate (sodium bicarbonate), and hydrogen peroxide. The antimicrobial characteristics of vinegar and lemon juice are related to the contents of acetic and citric acid, and these organic acids are added to food as protective agents (Fong et al., 2011; Ousaaid et al., 2021). Because the pH value of sodium bicarbonate in a neutral solution is balanced at a level close to 8.4, it is inadequate to inhibit the growth of most food-borne microorganisms that proliferate in an environment with a pH level of 9 - 10 (Yang et al., 2009; Fong et al., 2011).
The content and antimicrobial efficacy of vinegar vary according to the raw materials used because different fruits, in addition to grapes, are used for vinegar production. Kelebek et al. (2021) reported that the antimicrobial activity of grape vinegar is higher than that of apple vinegar and has a higher antioxidant capacity. Mulberry vinegar has more lactic and succinic acid content and high potential antioxidant and antimicrobial activity, which positively affect human health, as compared to other kinds of vinegar (Gündoğdu et al., 2018; Şengün & Kılıç, 2020). Acetic acid is the main component of vinegar and shows potent antimicrobial activity against fungi and bacteria (Budak, 2010; Öztürk et al., 2015; Kelebek et al., 2017; Benedek et al., 2022). Different types of vinegar produced by various fruits show different antimicrobial effects on distinct bacteria. For example, apple vinegar shows activity against Candida albicans, Staphylococcus aureus, Escherichia coli (Bakır et al., 2017; Kadiroğlu, 2018; Yagnik et al., 2018; Ousaaid et al., 2021), Bacillus subtilis DSMZ1971, Enterobacter aerogenes ATCC13048, L. monocytogenes ATCC7644, Pseudomonas aeruginosa DSMZ50071, Salmonella enteritidis ATCC13075, S. typhimurium SL1344, Staph. aureus ATCC25923, Staph. epidermidis DSMZ20044, Enterococcus faecium, Klebsiella pneumoniae, L. innocua, S. infantis (Baldas & Altuner, 2018; Kadiroğlu, 2018), and Pseudomonas aeruginosa (Ousaaid et al., 2021); white and red grape vinegar shows activity against Staph. aureus ATCC29213, E. coli ATCC25922, C. albicans ATCC10231 (Bakır et al., 2017; Antoniewicz et al., 2020), Bac. subtilis DSMZ1971, C. albicans DSMZ1386, Enterobacter aerogenes ATCC13048, Ent. faecalis ATCC29212, L. monocytogenes ATCC7644, P. aeruginosa DSMZ50071, P. fluorescens P1, S. enteritidis ATCC13075, S. typhimurium SL1344, Staph. aureus ATCC25923, S. epidermidis DSMZ20044, Ent. durans, Ent. faecium, K. pneumoniae, L. innocua, S. infantis, and S. Kentucky food isolate; pomegranate, balsamic, gilaboru, blackberry, lemon, rosehip, mulberry, apricot, rice, date, blueberries, hawthorn, and artichoke vinegar show activity against Staph. aureus, Streptococcus pyogenes, K. oxytoca, Ent. faecalis, Bac. cereus, Bac. subtilis, Erwinia carotovora, E. coli, and C. albicans (Bakır vd. (2017); white mulberry vinegar shows activity against Staph. aureus, Strep. pyogenes, K. oxytoca, Ent. faecalis, Bac. cereus, Bac. subtilis, Erwinia carotovora, E. coli, C. albicans (Aydın, 2013; Şengün & Kılıç, 2018, Şengün & Kılıç, 2020), L. monocytogenes Scott A, S. typhimurium NRRLB4420, and Pediococcus acidilactici ATCC8042 (Şengün & Kılıç, 2018, Şengün & Kılıç, 2020); and fig vinegar shows activity against Bac. subtilis ATCC6037 (Şengün & Kılıç, 2020). However, few studies have been conducted on the antimicrobial effects of different types of vinegar on L. monocytogenes. In the study of Nastou et al. (2012), lettuce, cucumber, and parsley were inoculated with L. monocytogenes and dipped in 0.5% - 2% acetic acid for 5 - 30 min. The decontamination efficiency of acetic acid varied according to the different vegetable species; 1% acetic acid treatment caused a 1 log CFU/cm2 reduction, while a reduction of 0.7 log CFU/cm2 was observed for cucumber. For parsley, the dose of 0.5% caused 1.2 log CFU/cm2 reduction, and the maximum reduction was recorded at 2.6 log CFU/cm2 with 2% acetic acid dose. In another study, the antimicrobial activity of different vinegar types in L. monocytogenes-inoculated lettuce was 2.15 ± 0.04 log CFU/mL for balsamic vinegar, 0.18 ± 0.06 log CFU/mL for white wine vinegar, and 1.13 ± 0.06 log CFU/mL for acetic acid solution as compared to washing with water (0.05 ± 0.04 log CFU/mL) (Ramos et al., 2014). In a study that evaluated the antimicrobial activity of hawthorn vinegar on pathogen microorganisms, including E. coli ATCC25922, Ent. faecalis ATCC29212, Campylobacter jejuni ATCC17028, L. monocytogenes ATCC19115, and Staph. aureus ATCC25923, it was found that the tested bacteria were sensitive to hawthorn vinegar, and the inhibition zone diameter ranged from 12.61 to 16.18 mm. Hawthorn vinegar showed the most potent effect on Campylobacter jejuni (16.18 mm) and L. monocytogenes (14.78 mm), whereas the least effect was observed on an Ent. faecalis (12.61 mm) strain (Özdemir et al., 2021).
Sodium bicarbonate (NaHCO3) has been used widely as a blowing agent in food products and as an antibacterial and antifungal agent in cattle feed and industrial products such as toothbrushes and mouthwashes. It can be used as a disinfectant as it has been classified as GRAS for humans and has low cost (Waple, 2017). Sodium bicarbonate was found to be effective in inhibiting E. coli contamination at the doses of 10 and 100 mM (Labaiden et al., 2013); moreover, the 10% dose was effective in reducing the growth of Staphylococcus, Bacillus, Proteus, and Klebsiella species (Al-Rawi et al., 2018). However, sodium bicarbonate showed low efficacy against food pathogens, including L. monocytogenes, E. coli O157:H7, and S. typhimurium (Yang et al., 2009). Kilonzo-Nthenge and Liu (2019) reported that the application of bicarbonate as an antimicrobial agent to S. enterica inoculated spinach leaves was unsuccessful in terms of reducing the pathogen load on fruits and vegetables. Hydrogen peroxide (H2O2) is widely used in food production plants, aseptic packaging, and surface disinfestation because of its disinfectant, antiviral, and antimicrobial properties (National Center for Biotechnology Information, 2022). A previous study showed complete inhibition of L. monocytogenes Scott A with the application of 3.0% H2O2 solution for 10 min during the daily disinfection process in the food operation area (Szendy, 2021). Zhang & Yang (2017) showed that the microbial load of fresh-cut lettuce was reduced significantly by a combination of 1% H2O2 and 0.6% citric acid. The authors also reported that the microbial load was reduced effectively by 4 mg/L electrolyzed water and 1% H2O2 combination, without any effect on the sensory quality; thus, these treatments were applicable in reducing microbial growth in fresh-cut lettuce.
Citric acid is present in lemon at the concentration of 5-6 g/100 mL, and it contains some antimicrobial bioactive compounds such as flavonoids, carotenoids, limonoids, tannin, and terpenoids (González-Molina et al., 2010; Ekawati & Darmanto 2019). The growth of Yersinia enterocolitica inoculated into carrot at the dose of 106 CFU/mL was inhibited by 100% lemon juice, 75% lemon juice, and a combination of lemon juice and vinegar (Şengün & Karapınar, 2005a). In a similar study, the combination of lemon juice and vinegar caused the maximum reduction of S. typhimurium inoculated into rocket. Likewise, lemon juice, vinegar, and their mixture showed 0.87 - 2.93, 0.66 - 2.92, and 0.86 - 3.24 log CFU/g reduction, respectively, in the count of S. typhimurium (Şengün & Karapınar, 2005b). The growth of S. enteriditis and E. coli inoculated at high doses in Cig Kofte was reduced by different doses of lemon juice treatment (Bingöl et al., 2011). However, another study reported that lemon juice treatment was not effective in reducing the load of E. coli O157: H7, S. enteritidis, and L. monocytogenes on beef (Yang et al., 2013).
The present study aimed to determine the reduction potential of the natural antimicrobial agents, namely lemon juice, sodium bicarbonate, hydrogen peroxide, and different types of traditional and commercial vinegar, against L. monocytogenes strains.
2. Materials and methods
L. monocytogenes strains and reference strain
Twenty-nine L. monocytogenes strains isolated from ready-to-eat foods and a standard L. monocytogenes ATCC7644 strain were used in this study. All the L. monocytogenes strains were identified at the molecular level and obtained from the culture collection of Department of Food Engineering, Faculty of Engineering, Ankara University.
Natural antimicrobial compounds
The antimicrobial agents, pH values, and the code systems used in this study are listed in Table 1. In the table, G, and T code means traditional and commercial vinegar products, respectively. Other antimicrobial agents such as carbonate, lemon, and hydrogen peroxide are given the D codes. Accordingly, eight types of vinegar produced by the traditional method under home conditions, and six types of commercial vinegar provided by different companies, carbonate, lemon, and hydrogen peroxide (Merck, Germany) were used. Traditional kinds of vinegars are prepared at homes from a variety of substrates under uncontrolled conditions and they are unpasteurized.
Preparation of stock cultures
The stock cultures of the L. monocytogenes strains provided by the culture collection and the reference strain were prepared using Brain Hearth Infusion (BHI) broth (Merck, Germany) and Tryptic Soy Broth (TSB) (Merck, Germany) with 20% sterile glycerol. The stock cultures were stored at -20 °C and were used as the study material after further culturing in TSB and/or BHI broth media at 37 °C for 18 h.
Preparation of the bacterial culture
The stock cultures were streaked on Tryptic Soy Agar (TSA) (Merck, Germany) to obtain isolated colonies. Single colonies selected after incubation at 37 °C for 20 - 24 h were transferred to 10 mL TSB and incubated under the same conditions. Three different concentrations of the bacterial cultures with cell density adjusted to 105, 106, and 107 CFU/mL after incubation were used for the antimicrobial susceptibility studies.
Natural antimicrobial agents used as a stress factor
Homemade and commercial vinegar types produced from different raw materials, lemon juice, sodium bicarbonate, and hydrogen peroxide, were used as stress factors to inhibit the growth of the L. monocytogenes strains. Different concentrations of these agents were tested against the L. monocytogenes strains with distinct cell densities. In this context, the following doses of the antimicrobial agents have been tested: 50% and 100% concentrations for traditional or commercial vinegar types and lemon juice; 10%, 15%, and 20% doses for sodium bicarbonate; and 10%, 20%, 30%, 40%, and 50% concentrations for hydrogen peroxide.
Comparison of antimicrobial agents and pathogens cultured under in vitro conditions
The disc diffusion method modified from Bauer et al. (1966) was used to determine the antimicrobial efficacy of the selected antimicrobial agents against the L. monocytogenes strains. Ten milliliters of TSB containing the culture at 1 × 105 CFU/mL or 1 × 106 CFU/mL or 1 × 107 CFU/mL cell density was inoculated on 90 mL TSA when the optimal temperature for plating was reached (45 °C). The agar medium was mixed homogeneously and distributed equally into four separate Petri plates. The antimicrobial agents sterilized by passing through a membrane filter (Sartorius) of 0.45 µm pore diameter were impregnated into 6-mm-diameter sterile discs (Oxoid Ltd., ES). The discs soaked with antimicrobial agents were kept at room temperature for 18 h. These discs were then placed on Petri plates with adequate care to avoid contact with each other. Four discs were placed on each Petri plate, and the plates were incubated at 37 °C for 24 h. After the incubation period, the resulting inhibition zone diameters were measured using a ruler. The antimicrobial susceptibility of L. monocytogenes strains against the antimicrobial agents was evaluated by measuring the inhibition zone diameter. The disc diffusion experiments were performed in duplicate. The strains were insensitive when the zone diameter was ≤ 6 mm, less sensitive for the zone diameter of 6.1 - 30 mm, and sensitive for the zone diameter of ≥ 31 mm. Sterilized distilled water was used as a negative control, and ampicillin (10 µg/disc) and gentamicin (10 µg/disc) were used as positive controls.
Statistical analysis
Measurements related to the quantitative variable were expressed as mean and standard deviation, and repeated measurements were analyzed by variance analysis (ANOVA). When the main effect of repeated measurements and interaction was not significant in the first test, the model was established with two factorial interaction models (bacteria concentration and natural antimicrobial compound) and tested. When the interaction was significant, the measurement average related to the natural antimicrobial compounds for each bacterial concentration was tested using the Bonferroni correction. All analyses were performed using SPSS (version 25) within 5% error limits.
3. Results and discussion
In the present study, the antimicrobial efficacy of eight types of vinegar produced from different fruits through the traditional homemade process was evaluated against L. monocytogenes strains (Figure 1).

Figure 1 Inhibition zones around the disc against the Listeria monocytogenes strain. (disc 1: Mulberry vinegard, disc 2 and 4: Negative control (distilled water), disc 3: Hydrogen peroxide)
The mean and standard deviation values of the antimicrobial activity of the test compounds against different bacterial concentrations are shown in Figure 2.
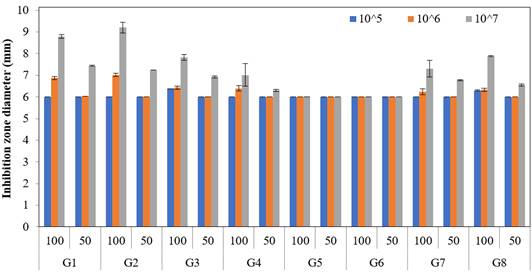
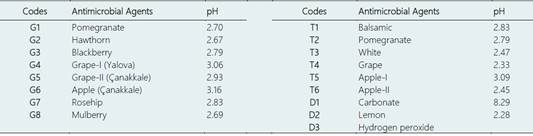
Figure 2 Comparison of the average values of inhibition zone diameters of natural antimicrobial agents against different bacterial concentrations (mm). Error bars show the differences among the treatments within 5% error limits. “G” refers to traditional vinegar, and the description is given in Table 1. The numerals 50 and 100 refer to the “%” concentration of vinegar.
As shown in Figure 2, 100% concentration of the traditional vinegar sample produced from pomegranate (G1) was insensitive (6 ± 0.3 mm) against 105 CFU/mL, whereas it was less sensitive against 106 and 107 CFU/mL concentrations of L. monocytogenes strains (6.9 ± 0.3 and 8.8 ± 0.3 mm, respectively). The 50% concentration of the antimicrobial agent was insensitive (6 ± 0.3 mm) against 105 and 106 CFU/mL bacterial concentrations but was less sensitive against 107 CFU/mL concentration of L. monocytogenes (7.4 ± 0.3 mm). Similar results were noted for hawthorn vinegar (G2) for both 100% and 50% concentrations against the different doses of L. monocytogenes strains. Regarding the antimicrobial efficacy of blackberry vinegar (G3), 100% concentration was less sensitive (6.4 ± 0.3, 6.4 ± 0.3, and 7.8 ± 0.3 mm) to 105, 106, and 107 CFU/mL doses of the L. monocytogenes strain, respectively, but the 50% concentration of G3 was insensitive against 105 and 106 CFU/mL doses and less sensitive to 107 CFU/mL (6.9 ± 0.3 mm) dose. Likewise, 100% of G4 was found to be insensitive to 105 CFU/mL dose of L. monocytogenes, whereas it was less sensitive (6.4 ± 0.3 and 7 ± 0.3 mm, respectively) against 106 and 107 CFU/mL doses. However, a 50% concentration of G4 was insensitive to 105 and 106 CFU/mL doses and less sensitive (6.3 ± 0.3 mm) against 107 CFU/mL doses Unlike the above vinegar types, G5 was insensitive at both 100% and 50% concentrations to all three doses of the L. monocytogenes strains. G4 and G5 vinegar types were insensitive against L. monocytogenes in general, and the highest zone diameter measured was 7 ± 0.3 mm. In contrast, Öztürk et al. (2015) found that the inhibition zone diameter of homemade grape vinegar was 10.18 - 30.71 mm. Thus, this result did not agree with the present study. The difference may be due to the different grape varieties and vinegar production techniques used in the two studies. Similar to G5, the G6 vinegar type (apple vinegar) was also insensitive at both 100% and 50% concentrations against all the doses of the L. monocytogenes strains. Rosehip vinegar (G7) at 100% con centration was insensitive to the 105 CFU/mL dose but less sensitive (6.2 ± 0.3 and 7.3 ± 0.3 mm, respectively) against the 106 and 107 CFU/mL L. monocytogenes doses. The 50% concentration of this antimicrobial agent was insensitive to 105 and 106 CFU/mL doses but less sensitive (6.8 ± 0.3 mm) against the 107 CFU/mL dose. Mulberry vinegar (G8) at 100% concentration was less sensitive (6.3 ± 0.3, 6.3 ± 0.3, and 7.9 ± 0.3 mm, respectively) to all concentrations of L. monocytogenes, but it was insensitive against 105 and 106 CFU/mL doses and less sensitive (6.6 ± 0.3 mm) to the 107 CFU/mL dose at 50% concentration. These results are supported by the study of Şengün & Kılıç (2018), which found no inhibition effect of mulberry vinegar against L. monocytogenes strains.
The results revealed that the antimicrobial efficacy of the eight types of traditional vinegar used as a natural antimicrobial agent against the different doses of L. monocytogenes was significant at p < 0.05. Moreover, the traditional vinegar samples showed higher antimicrobial efficacy against L. monocytogenes at 100% concentration than at 50% concentration. The antimicrobial activity of 100% and 50% concentrations of commercial vinegar types against L. monocytogenes strains is shown in Figure 3.
Both 100% (7.5 ± 0.3, 8.7 ± 0.3, and 10.5 ± 0.3 mm, respectively) and 50% (6.2 ± 0.3, 6.3 ± 0.3, and 7.5 ± 0.3 mm) concentrations of commercial balsamic vinegar (T1) were less sensitive to all L. monocytogenes strains. Similar results were noted for pomegranate vinegar (T2), which was less sensitive to all L. monocytogenes strains at both concentrations. Contrary to the results of the present study, Öztürk et al. (2015) reported that the pomegranate vinegar inhibited L. monocytogenes, and the inhibition zone diameter was measured as 15.62 mm. Similar to the 100% concentration of balsamic and pomegranate vinegar, the white vinegar (T3) was less sensitive (8.4 ± 0.3, 10.4 ± 0.3, and 11.3 ± 0.3 mm) against all the doses of L. monocytogenes strains. However, the 50% concentration was insensitive to the 105 CFU/mL dose but less sensitive (6.8 ± 0.3 and 7.5 ± 0.3 mm) against 106 and 107 CFU/mL doses. Furthermore, the 100% concentration of T4 was less sensitive (6.3 ± 0.3, 8.1 ± 0.3, and 8.4 ± 0.3 mm) to all L. monocytogenes strains; however, the 50% dose was insensitive against 105 and 106 CFU/mL doses and less sensitive (6.2 ± 0.3 mm) to the 107 CFU/mL dose.
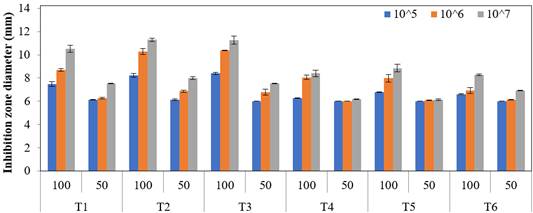
Figure 3 Comparison of the average values of inhibition zone diameter of natural antimicrobial agents against different bacterial concentrations (mm). Error bars show the differences among the treatments within 5% error limits. “T” refers to traditional vinegar, and the description is given in Table 1. The numerals 50 and 100 refer to the “%” concentration of vinegar.
The inhibition zone diameters of commercial grape vinegar ranged from 6.0 to 8.4 mm. These results are inconsistent with those of Öztürk et al. (2015), who reported that the inhibition zone diameter of commercial grape vinegar against L. monocytogenes was 17.73 mm. These differences may be due to the differences in the variety of grape used for vinegar production or variation in the vinegar producing process. Commercial apple vinegar (T5) showed a similar result to grape vinegar for 100% concentration and was less sensitive against all the doses of L. monocytogenes strains (6.8 ± 0.3, 8 ± 0.3, and 8.9 ± 0.3 mm, respectively, for 105, 106, and 107 CFU/mL). At the 50% concentration, T5 was insensitive to the 105 CFU/mL dose of L. monocytogenes, whereas it was less sensitive against 106 and 107 CFU/mL doses (6.1 ± 0.3 and 6.2 ± 0.3 mm). The 100% concentration of the other commercial apple vinegar type (T6) was also less sensitive to all L. monocytogenes strains like T5. Moreover, the inhibition zone diameters for the 105, 106, and 107 CFU/mL doses were 6.6 ± 0.3, 6.9 ± 0.3, and 8.3 ± 0.3 mm, respectively. Like T5, the 50% concentration of T6 was insensitive to the 105 CFU/mL dose of L. monocytogenes strains and less sensitive (6.1 ± 0.3 and 6.9 ± 0.3 mm, respectively) to 106 and 107 CFU/mL doses. The commercial apple vinegar types coded T5 and T6 were sensitive against L. monocytogenes strains, and the highest zone diameter was 8.9 ± 0.3 mm. Contrary to this result, Öztürk et al. (2015) reported the inhibition zone diameter of commercial apple vinegar as 17.51 mm against L. monocytogenes. This difference might be due to the distinct origin of apples used to produce vinegar.
The differences in the antimicrobial efficacy of the commercial and traditional vinegar types against L. monocytogenes were statistically significant (p < 0.05). Moreover, the commercial vinegar types showed higher antimicrobial activity against L. monocytogenes than the traditional ones. Various researchers (Nastou et al., 2012; Öztürk et al., 2015; Bakır et al., 2017; Baldas & Altuner, 2018; Şengün & Kılıç, 2018; Şengün & Kılıç, 2020) have shown differences in the antimicrobial activity between homemade and commercial vinegar against L. monocytogenes strains, similar to the findings of the present study. In the present study, the inhibition zone diameter of both commercial and traditional vinegar samples against L. monocytogenes was lower (Figure 2 and Figure 3) than that reported by Öztürk et al. (2015) for conventional vinegar (10.18 - 30.71 mm) commercial vinegar (15.62 - 18.05 mm) and by Şengün et al. (2018) for commercial mulberry vinegar against L. monocytogenes (13.5 mm).
The antimicrobial activities of different concentrations of the other antimicrobial agents such as carbonate, lemon, and hydrogen peroxide against L. monocytogenes are shown in Figure 4.
It was found that the 10%, 15%, and 20% concentrations of carbonate (D1) were insensitive to 105, 106, and 107 CFU/mL of L. monocytogenes. Yang et al. (2009) reported that a bicarbonate solution causes < 1 log reduction in the count of L. monocytogenes, and there is no inhibition effect of carbonate on food pathogen. This result supports the findings of the present study. The 100% concentration of fresh lemon samples (D2) was less sensitive to all the doses of L. monocytogenes strains, whereas the 50% concentration was insensitive. The antimicrobial efficacy of hydrogen peroxide (D3) was higher than that of the other two (D1 and D2) antimicrobial agents and varied from 33.9 mm (10%) to 51.9 mm (50%). Moreover, the 10%, 20%, 30%, 40%, and 50% concentrations of D3 were sensitive to all the doses of L. monocytogenes (Fig. 4). The antimicrobial efficacy of different concentrations of carbonate, lemon, and hydrogen peroxide against the different doses of L. monocytogenes strains was significant at the level of p < 0.05. The antimicrobial activity of hydrogen peroxide against L. monocytogenes was higher than that of the other antimicrobial agents tested in this study.
Figure 4 Comparison of the average values of inhibition zone diameter of natural antimicrobial agents against different bacterial concentrations (mm). Error bars show the differences among treatments within 5% error limits. “D” refers to traditional vinegar, and the description is given in Table 1. The numerals 50 and 100 refer to the “%” concentration of vinegar.
4. Conclusions
The inhibitory effect of homemade and commercial vinegar types, lemon, sodium bicarbonate, and hydrogen peroxide, against the growth of L. monocytogenes strains was investigated in this study. Several factors such as origin, environmental conditions, production methods, processing, and storage conditions are affected the quality of vinegar. Vinegar products could be used as a natural sanitizer in both the food industry and home conditions. Moreover, they can be also used as a bioactive ingredient in the food industry. Obtained results revealed that both traditional and commercial vinegar showed the highest antimicrobial efficacy when used undiluted (100% concentration). The antimicrobial activity of commercial vinegar types such as balsamic, pomegranate, white, and grape against L. monocytogenes was higher than that of apple vinegar. Moreover, commercial vinegar types showed significantly higher antimicrobial efficacy than their traditional counterparts. However, the inhibition zone diameters of both commercial and traditional vinegar types against L. monocytogenes were lower than those reported previously. Furthermore, of the other tested antimicrobial agents, hydrogen peroxide showed the highest antimicrobial activity against L. monocytogenes. The antibacterial results obtained in this study also show that vinegar products are important in terms of revealing the potential of using vinegar in the treatment of infectious diseases.