1. Introduction
The amaranth (Amaranthus spp. L.) has a pre-Hispanic origin of approximately 8,000 years B.C. in Central and South America (Thapa & Blair, 2018), having been domesticated, cultivated and used for more than 4,000 years in America, from where it probably spread to other parts of the world. This domestication began with the appearance of pale ivory or white seeds instead of glossy blackish seeds typical of wild species, which has been confirmed by archaeological (Sauer, 1976) and molecular (Stetter et al., 2020) evidence. Archaeological data also reveal that the seeds and leaves were widely used for food by the pre-Hispanic inhabitants (Peralta et al., 2008), having been cultivated together with corn, beans, potatoes, quinoa or pumpkin by the Aztecs in the Valley of Mexico, by the Mayans in Guatemala and by the Incas in South America, in Peru, Bolivia, and Ecuador (Sauer, 1967).
Amaranth belongs to the family Amaranthaceae Juss., which includes 65-75 genera and about 900 species (Robertson, 1981; Müller & Borsch, 2005). The type genus is Amaranthus L., which includes about 70 species, essentially distributed in tropical and subtropical regions, of which about 60 are native to the American continent and only about 15 come from Europe, Asia, Africa and Australia (Sauer, 1967; Stetter & Schmid, 2017; Waselkov et al., 2018). In the American continent, the most important amaranth species are the three species cultivated for grain production (A. caudatus L., A. cruentus L. and A. hypochondriacus L.), together with A. hybridus L., A. quitensis Kunth and A. powellii S. Wats., which are usually considered as possible wild parents of the former (Sauer, 1967; Kietlinski et al., 2014; Gonçalves-Dias & Stetter, 2021; Thapa et al., 2021). A. quitensis is only distributed in South America, being considered a semi-cultivated species, so it would be in an incipient state of domestication (Sauer, 1967). In Ecuador, it is traditionally known by the name of ataco, sangorache or black amaranth, whose plant is usually red or purple in colour and produces black seeds.
Andean grain pseudocereals are foods with significant protein content, specifically, the ataco seed contains 13% to 17% of the dry weight, which are values significantly higher than those normally found in most cereals. In addition, they are high quality proteins, since they have an adequate balance of essential amino acids, mainly lysine, methionine and tryptophan (Peralta et al., 2012;2013). Farmers in the Ecuadorian highlands use ataco or sangorache as a natural medicine through infusions of leaves and inflorescences (Peralta et al., 2008; Patrimonio Alimentario, 2013). On the other hand, the inflorescence is used to obtain dyes for the production of traditional beverages, such as "colada morada", "horchata" or "draque" (Peralta et al., 2008; Patrimonio Alimentario, 2013). Likewise, the black amaranth grain is beginning to be incorporated into Ecuadorian gastronomy for the preparation of typical foods (Michelle, 2015). The ataco is also used as an ornamental plant in gardens and parks, because its inflorescence has showy coloration and varied shapes (Peralta et al., 2008; Suquilanda, 2011).
The ataco is usually cultivated throughout the Ecuadorian highlands at altitudes ranging from 2,000 to 3,000 m.a.s.l., found alone or associated with other crops (Peralta, 2009). Currently, there are no data on the cultivated area and the production of amaranth in Ecuador. Only partial data is available for some provinces, such as Imbabura, where the majority of amaranth producers are located in the cantons of Cotacachi, Otavalo and Antonio Ante, with just over 15 cultivated hectares and a annual grain production close to 50,000 kg, of which 90% corresponds to the improved INIAP-Alegría variety and only the remaining 10% to landraces of ataco or sangorache (Jurado, 2019). On the other hand, in Ecuador, as in the rest of the countries of the Andean region, the native varieties of traditional crops, among which the ataco would be, seem to be suffering in recent decades a process of genetic erosion due essentially to factors such as reduction of agricultural production to few crops, changes in eating habits, low product prices, inadequate access routes or unfavorable marketing policies, among others (INIAP-DENAREF, 2016). Thus, currently there are few provinces of the Ecuadorian highlands in which this crop is still maintained, having been reduced in a good part of them, as is the case of the provinces of Pichincha, Azuay and Loja (Personal observation), in which a significant number of accessions were collected in the early 1980s (Mazón et al., 2003).
Know the phenotypic diversity of local accessions of cultivated species is of great importance for the correct conservation and management of these plant genetic resources. In the case of amaranth, there are few studies on the agro-morphological characterization of landraces. Thus, in the Asian continent, evaluations of reproductive traits have been carried out and, especially, those related to yield in amaranth cultivars and genotypes from India (Parveen et al., 2013; Malaghan et al., 2018). Likewise, Gueco et al. (2016) carried out a study of morphological diversity in native accessions of amaranth from different places in the Philippines. In the African continent, there are studies on the agro-morphological characterization of local accessions of amaranth from Nigeria (Oboh, 2007), Tanzania (Mbwambo et al., 2015) or Malawi (Nyasulu et al., 2021), as well as South African genotypes (Gerrano et al., 2015;2017). In the American continent, the works of Ruiz Hernández et al. (2013) and Thapa & Blair (2018) stand out. In the first, they analyzed the morphological variability of 155 accessions of different cultivated species, essentially grain amaranths (A. hypochondriacus, A. cruentus and A. caudatus), conserved in the Germplasm Bank of the National Institute of Forest, Agricultural and Livestock Research (INIFAP; Mexico). In the second, they evaluated the morphological diversity in 208 accessions belonging to nine different species of Amaranthus (including A. quitensis) from the United States Department of Agriculture (USDA).
In Ecuador, between 1981 and 1986, the National Institute for Agricultural Research (INIAP) carried out several expeditions to collect landraces of black amaranth (A. quitensis) grown in the Ecuadorian highlands, which were preserved in the Germplasm Bank of the INIAP (Mazón et al., 2003). The collection increased to more than 400 accessions of Amaranthus spp. with germplasm from international exchanges, essentially from other cultivated species. In a first stage of agro-morphological characterization, these accessions were studied in order to identify them taxonomically, as well as to select promising lines for future breeding programs based on yield and earliness (Mazón et al., 2003). More recently, all this information on morphological diversity has been used to try to generate a core collection that contains the greatest possible variability in a reduced number of accessions (Tapia et al., 2017). Currently, for ataco, as for most Andean crops, there are no data on the situation (diversity and conservation status) in which the landraces are found. For this reason, to carry out this study, it was first proposed to collect germplasm of ataco traditional varieties in the three Andean provinces that currently have a greater representation of this ancestral crop (Imbabura, Tungurahua and Cañar).
The main objective of the study was to evaluate the phenotypic diversity existing in black amaranth cultivated in the Ecuadorian Andean region, for which the agro-morphological characterization of the accessions of ataco landraces collected at different times would be carried out in three representative Andean provinces for this crop. On the other hand, it is intended to carry out a comparative study between the phenotypic diversity existing in accessions collected at the beginning of the 80s and the diversity present in accessions collected more recently, in order to know the diversity variation that may have occurred in the last decades. This work would be one of the few studies in which a comparative study has been carried out between accessions collected at different times, which would allow knowing how is being the management and on-farm conservation of the ataco landraces by the farmers of the Andean region of Ecuador.
2. Materials and methods
2.1 Plant material
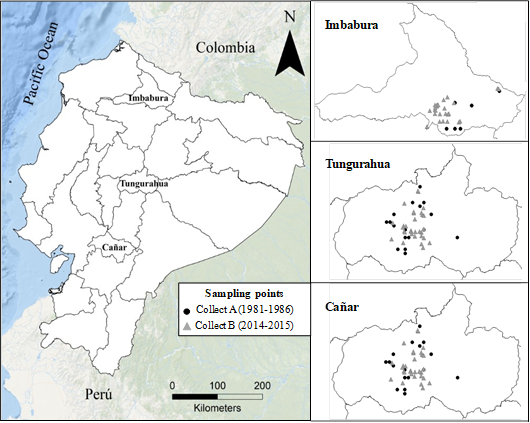
A total of 139 ataco (Amaranthus quitensis Kunth) land races accessions were analyzed in this study, which were collected at two different times in three representative provinces of the Ecuadorian Andean region for this crop, in terms of cultivated area, production, consumption and economic importance (Nieto, 1989; Mazón et al., 2003): Imbabura, Tungurahua and Cañar. The first collect (named "Collect A") was carried out between 1981 and 1986, and 50 accessions were collected (A1 to A50): 9 in Imbabura, 24 in Tungurahua and 17 in Cañar (Supplemen tary Material 1; Figure 1). The second collect (named "Collect B") was carried out in 2014 and 2015, collecting 89 accessions (A51 to A139): 29 in Imbabura, 31 in Tungurahua and 29 in Cañar (Supplementary Material 1; Figure 1). The 139 accessions are conserved in the Germplasm Bank of the National Institute for Agricultural Research (INIAP) located in Pichincha, Ecuador.
2.2 Agro-morphological evaluation
The assay for agro-morphological characterization was conducted throughout 2016, under field conditions of the Tunshi Experimental Station at the Higher Polytechnic School of Chimborazo (ESPOCH), located in Riobamba canton, Licto parish (-1º44’54’’ South Latitude, -78º37’33’’ West Longitude; altitude 2710 m.a.s.l.), with an annual average temperature of 13.9 ºC, annual precipitation of 544.1 mm, and sandy loam soil type with a pH of 7.2. The land preparation was mechanized in all plots and 6 m furrows in length were made. Fourteen plants of the same accession were planted in each furrow, with a distance of 0.40 m between plants and 0.80 m between furrows, and rye (Secale cereale L.) was planted as a living barrier between furrows.
Table 1 Quantitative descriptors (D1-D11) used for agro-morphological characterization of 139 ataco (A. quitensis) accessions
| Code-Descriptor | Unit of measure |
|---|---|
| D1-Plant height | cm |
| D2-Petiole length | cm |
| D3-Leaf length | cm |
| D4-Leaf width | cm |
| D5-Inflorescence length | cm |
| D6-Seed size | mm |
| D7-Grain yield per plant | g |
| D8-Days to emergency | number |
| D9-Days to floral bud formation | number |
| D10-Days to flowering | number |
| D11-Days to physiological maturity (or days to harvest) | number |
Agronomic practices were the same for all plots, and they were similar to those used by the farmers who cultivate ataco landraces on Andean region farms. To carry out the agro-morphological characterization of each accession, 10 of the 14 plants were taken at random, and 30 descriptors selected from Sumar et al. (1986) were evaluated, 11 of them for quantitative traits (descriptors D1 to D11; Table 1) and 19 for qualitative traits (descriptors D12 to D30; Table 2), covering different phenological stages of the plant.
2.3 Data analysis
Statistical analysis of the agro-morphological data was carried out considering accessions from different collects, from different provinces for the same collection, or from the same province for different collects. Data of quantitative variables (D1 to D11) were subjected to descriptive statistical analysis (maximum, minimum, mean, standard deviation, and coefficient of variation) along with an analysis of variance, and means of different groups of accessions were compared using Fisher’s Least Significant Difference (LSD) test (Fisher, 1936) to determine significant differences among them. To find out correlations between two quantitative variables, a Pearson’s correlation analysis (Conover, 1999) was performed. Data from qualitative variables (D12 to D30) were used to construct a table of frequencies. Polymorphic qualitative traits more discriminating was identified using the Chi-square test (Cochran, 1954), and Pearson’s (C) and Cramér’s V contingency coefficients (Pearson, 1922; Cramér, 1946). To establish correlations between two qualitative variables, Spearman’s coefficients (Spearman, 1904) were calculated. All these analyses of the agro-morphological data were performed using the InfoStat program version 2020 (InfoStat, 2020).
Data from quantitative and polymorphic qualitative variables were subjected to multivariate analysis by using Gower’s distance (Gower, 1967) and Ward’s hierarchical clustering method (Ward, 1963) to construct a dendrogram to show the phenotypic relationships among ataco accessions using the InfoStat program. A co-phenetic matrix was derived from the Gower distances matrix to test the goodness-of-fit of the clusters by comparing the two matrices using the Mantel matrix correspondence test in the MxComp program of the NTSYS-pc package version 2.2 (Rohlf, 2008), using 10,000 random permutations. Also, a statistical analysis to assess more discriminating quantitative and qualitative traits among clusters defined in the dendrogram was carried out. Means of quantitative traits from different groups were compared using Fisher’s LSD test. Qualitative traits from different clusters were compared using the Chi-square test.
3. Results and discussion
3.1 Diversity in quantitative traits
Table 3 shows the results that have been obtained in the descriptive analysis for the 11 quantitative variables analyzed in the 50 accessions of ataco or sangorache (Amaranthus quitensis Kunth) of the Collect A (1981 to 1986) and the 89 of the Collect B (2014 and 2015), comparing both collects. Most of the quantitative traits analyzed showed high levels of variability, with coefficients of variation (CV) greater than 10%. The character grain yield per plant (D7) showed the highest level of variation in the analyzed accessions, both in Collect A (CV = 31.34%) and in Collect B (CV = 47.79%), while the number of days to physiological maturity (D11) presented the lowest levels of variation in both collects (CV = 0% and CV = 2.17%, respectively; Table 3). Similar results, with a high value of the coefficient of phenotypic variation for the character grain yield per plant and a low value of said coefficient for the number of days to physiological maturity, have been reported by Malaghan et al. (2018) when analyzing the variability in amaranth germplasm cultivated in India.
Table 2 Qualitative descriptors (D12-D30) used for agro-morphological characterization of 139 ataco (A. quitensis) accessions
| Code-Descriptor | States |
| D12-Growth habit | 1 Erect; 2 Prostrate |
| D13-Branching index | 1 No branching; 2 Few branches near the base of the stem; 3 Many branches near the base of the stem; 4 Branches all along the stem |
| D14-Stem pubescence | 0 Absent; 1 Present |
| D15-Stem pigmentation | 1 Green; 2 Purple; 3 Pink; 4 Red; 5 Yellow; 6 Other |
| D16-Spines in leaf axils | 0 Absent; 1 Present |
| D17-Leaf pubescence | 0 Absent; 1 Present |
| D18-Leaf colour | 1 Entire lamina pink or purple; 2 Basal area pigmented; 3 Stained with various colours; 4 Margin and veins pigmented; 5 Pale green or chlorotic; 6 Normal green; 7 Dark green; 8 Other |
| D19-Leaf shape | 1 Lanceolate; 2 Elliptic; 3 Ovoid; 4 Cuneate; 5 Rhombic; 6 Oval; 7 Oblong; 8 Other |
| D20-Leaf margin | 1 Entire; 2 Serrate; 3 Undulate; 4 Other |
| D21-Presence of prominent veins | 0 Absent; 1 Present |
| D22-Petiole section shape | 1 Grooved; 2 Circular; 3 Angular; 4 Other |
| D23-Root type | 1 Simple taproot; 2 Succulent taproot; 3 Other |
| D24-Inflorescence shape | 1 Compact; 2 With short branches (semicompact); 3 With long branches (semilax); 4 Lax; 5 Other |
| D25- Inflorescence attitude | 1 Erect; 2 Decumbent; 3 Semierect |
| D26-Presence of axillary inflorescence | 0 Absent; 1 Present |
| D27-Inflorescence colour | 1 Yellow; 2 Green; 3 Pink; 4 Red; 5 Purple; 6 Other |
| D28-Seed colour | 1 Yellow; 2 Pink; 3 Red; 4 Brown; 5 Black; 6 White; 7 Other |
| D29-Seed shape | 1 Lenticular; 2 Ellipsoid; 3 Ovoid; 4 Other |
| D30-Tumbling resistance | 1 Susceptible; 2 Low resistant; 3 Resistant |
Table 3 Maximum (Max) and minimum (Min) values, mean, standard deviation (SD) and coefficient of variation (CV) obtained for the 11 quantitative traits (D1 to D11) analyzed in the 50 ataco (A. quitensis) accessions collected between 1981 and 1986 (Collect A) in the provinces of Imbabura, Tungurahua, and Cañar, and in the 89 accessions collected in 2014 and 2015 (Collect B) in the same provinces
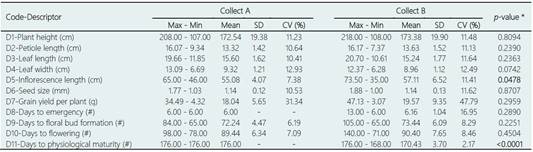
* p-values obtained in the analysis of variance using Fisher’s LSD test for each of the quantitative traits studied. The significant differences (p < 0.05) between means obtained for both collections are shown in bold.
Considering the 139 accessions as a whole, it is worth mentioning that high values of grain yield per plant (> 30 g) were obtained in 13 of them, of which only one corresponds to Collect A (A39; Cañar) and the remaining 12 to Collect B, being able to stand out among the latter accessions A96 (Tungurahua) and A138 (Cañar) for presenting the highest yield values, 47.13 g and 41.23 g respectively (see Supplementary Material 2). Characters related to precocity and yield are usually the most relevant for plant breeders (Tapia et al., 2017); in this sense, it was noticed that the accessions analyzed in the present work showed an important variation in terms of grain yield per plant, which may be interesting to take into account for future programs to improve the ataco cultivated in Ecuador. A high variability for the character grain yield per plant has also been detected in South African amaranth genotypes (Gerrano et al., 2015), as well as in cultivars from India (Parveen et al., 2013), which was considered great importance for its use in future programs for selection and improvement of cultivars of amaranth in both countries.
When both collects are compared, significant differences between them were only detected for two of the 11 quantitative variables studied (Table 3). On the one hand, the inflorescence length (D5) presented slight significant differences (p = 0.0478) between the two collects, with a mean length greater in the accessions of Collect B (Table 3). The largest inflorescence length was found in accession A61 (73.50 cm; Imbabura-Collect B) and the lowest value was found in accession A105 (35.00 cm; Tungurahua-Collect B) (see Supplementary Material 2). On the other hand, the variable days to physiological maturity or days to harvest (D11) showed highly significant differences (p < 0.0001) between both collects. The accessions collected between the years 1981 and 1986 presented a higher mean number of days to physiological maturity than the accessions collected in 2014 and 2015 (Table 3). All the accessions of Collect A were harvested at 176 days, while in Collect B 70% (62/89) of the accessions were earlier (with 168 days to harvest) than the remaining 30% (27 /89; 176 days to harvest) (see Supplementary Material 2). This greater precocity detected in accessions from the most recent collect could be due to the fact that the farmers have carried out a selection of the more precocious plants in the last decades, in order to obtain two harvests a year and thereby achieve a higher yield of this cultivation (Patrimonio Alimentario, 2013).
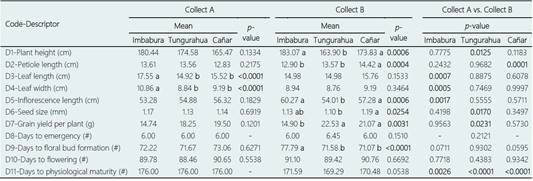
Table 4 shows the comparisons between the mean values obtained for each of the quantitative characters analyzed both in the different provinces for each of the two collects and in the same province for different collects. In Collect A, only significant differences (p < 0.0001) were detected in the descriptors related to leaf size, D3 (leaf length) and D4 (leaf width), so that the accessions collected in Imbabura province had a significantly larger mean leaf size than accessions from Tungurahua and Cañar provinces (Table 4). On the other hand, significant differences were found between provinces for six of the 11 characters analyzed in Collect B: plant height (D1), petiole length (D2), inflorescence length (D5), seed size (D6), grain yield per plant (D7) and days to floral bud formation (D9) (Table 4). It should be noted that the variable grain yield per plant was significantly (p = 0.0031) lower in Imbabura (14.90 g) than in the provinces of Tungurahua (22.53 g) and Cañar (21.07 g) (Table 4), which could be explained by the fact that in the province of Imbabura, unlike the other two provinces, it is common for local farmers to carry out an agroecological or organic production system for all Andean crops (Cevallos et al., 2020), with which lower yields are usually generated than those obtained with conventional agriculture systems (Seufert et al., 2012; Röös et al., 2018).
When the mean values obtained for the same province but in different collects were compared, again clearly significant differences were observed in the variable days to physiological maturity for the three provinces, being always more precocious the accessions of Collect B (Table 4). In the province of Imbabura, significant differences were also found between the two collects for the characters leaf length, leaf width, and inflorescence length; so that, in the most recent accessions, the mean values of the first two characters have decreased while that of the inflorescence length has increased (Table 4). Ruiz Hernández et al. (2013), analyzing the morphological variability of 155 accessions of different cultivated grain species of Amaranthus (A. hypocondriacus, A. cruentus and A. caudatus) conserved in the Germplasm Bank of the National Institute of Forest, Agricultural and Livestock Research (INIFAP; Mexico), also found a highly significant and negative association between leaf width and inflorescence length, so that the larger the inflorescence length, the smaller the leaf width. The increase in the length of the inflorescence in the Imbabura accessions could be explained by the fact that farmers in this province have been able to carry out a selection in recent decades in favor of plants with a larger inflorescence, since the inflorescence of ataco has been and is considered of great importance by the indigenous population of this province, as it is habitually used for the elaboration of “colada morada”, a very typical drink of the area (Peralta et al., 2008). In this sense, indigenous farmers from various locations in Mexico have also carried out an artificial selection in various cultivated amaranths in order to obtain a larger size of the inflorescence, in this case with the aim of achieving greater seed production (Matías et al., 2018).
When comparing the accessions of both collects in the province of Tungurahua, significant differences were detected for characters grain yield per plant, which was higher in the accessions of Collect B, and plant height, higher in the accessions of Collect A (Table 4). Ruiz Hernández et al. (2013) also observed a highly significant and negative correlation between plant height and grain yield in accessions of different cultivated species of amaranth, so that, when there was a greater vegetative or biomass production (e.g., plant height), the yield in amaranth it decreases, and vice versa. Similar results were also obtained by Nyasulu et al. (2021) in the agro-morphological characterization of Malawi amaranth accessions. The increase in yield detected in the province of Tungurahua could be due to the fact that at the beginning of the 1980s the ataco or sangorache was cultivated in isolation in small family gardens (chakras), or else it grew spontaneously among the corn or in the gardens of the farmers' houses (INIAP, 1985); while, in recent decades, the cultivation of sangorache in this province has been usually carried out in association with other crops, such as tree tomato, corn, beans, citrus, forage legumes or other vegetables (Patrimonio Alimentario, 2013; Personal observation during the germplasm collection carried out in 2014 and 2015), whose agronomic management is probably beneficial to generate an increase in the yield of the ataco as an associated crop (Stomph et al., 2020).
In relation to the province of Cañar, practically no significant differences were found for quantitative characters between the accessions of both collects (Table 4), which could be due to the fact that in this province the sangorache continues to be cultivated in a traditional way, without major changes in recent decades: in chakras and with a very small number of plants (Personal observation during the germplasm collection carried out in 2014 and 2015).
3.2 Diversity in qualitative traits
The Pearson’s correlation matrices (and their corresponding p-values) between the quantitative polymorphic characters analyzed in the 50 accessions of Collect A and in the 89 accessions of Collect B, are shown in Supplementary Material 3 and 4 respectively. In both cases, high absolute correlation values (> 0.70) and highly significant (p < 0.0001) were obtained between the characters leaf length (D3) and leaf width (D4) (Collect A = 0.937; Collect B = 0.896), and between days to floral bud formation (D9) and days to flowering (D10) (Collect A = 0.854; Collect B = 0.736), with the rest of the correlations being less than 0.5 and, in general, not significant. The existence of a strong positive and highly significant correlation between leaf length and width has also been detected in both South African amaranth genotypes (Gerrano et al., 2015) and Nigerian accessions of A. hybridus (Oboh, 2007). As for the characters days to floral bud formation and days to flowering, together with the character days to physiological maturity or to harvest, they are all descriptors related to the precocious of the accessions (Tapia et al., 2017), so it seems logical that they show a high and significant correlation between them.
Table 4 Comparison of mean values obtained for the 11 quantitative traits (D1 to D11) analysed in the ataco (A. quitensis) accessions belonging to different provinces for the same collect and in the same province for different collects. The p-values obtained by the analysis of variance for each descriptor studied are indicated; the significant differences (p < 0.05) between means are shown in bold. For each trait, different letters indicate significant differences (p < 0.05) between provinces of the same collect
On the other hand, it is worth mentioning that an important negative and significant correlation was detected again, at least in the most recently collected samples, between the characters plant height and grain yield per plant (Ruiz Hernández et al., 2013).
The analysis of the 19 qualitative characters studied in the 139 accessions of ataco, allowed to obtain the total frequencies and by provinces for each of the two collects. Twelve of the 19 characters analyzed were monomorphic in the accessions of both collects, and two more of the remaining seven were also monomorphic for Collect A (Table 5). That is, all the ataco accessions analyzed presented the same traits for the following qualitative descriptors: D12-growth habit (erect), D14-stem pubescence (absent), D16-spines in leaf axils (absent), D17-leaf pubescence (absent), D19-leaf shape (oblong), D20-leaf margin (entire), D21-presence of prominent veins (absent), D22-petiole section shape (grooved), D23-root type (simple taproot), D26-presence of axillary inflorescence (absent), D28-seed colour (black) and D29-seed shape (lenticular). The accessions of Collect A were also uniform for the descriptors: D13-branching index (branches all along the stem) and D30-tumbling resistance (resistant) (Table 5; see Supplementary Material 5). Unlike the quantitative agro-morphological characters, the analysis of the qualitative characters revealed a reduced phenotypic diversity in the ataco landraces cultivated in these Andean provinces of Ecuador. The interesting thing about these results is that these monomorphic characters would be defining the general qualities of the amaranth phenotype that has been cultivated and is cultivated in a traditional way in the Ecuadorian highlands.
Regarding the seven descriptors that presented some type of polymorphism in the set of 139 accessions, branching index (D13) and tumbling resistance (D30) showed the least level of variability, they were practically monomorphic, since in both cases it was found that 99.3% (138/139) of the accessions presented branches all along the stem and were resistant to tumbling, and only one accession for each character showed a different characteristic: A78 (Imbabura-Collect B) presented many branches near the base of the stem, while A69 (Imbabura-Collect B) was low resistant to tumbling (Table 5; see Supplementary Material 5).
Similarly, Thapa & Blair (2018) did not detect significant differences between clusters of amaranth accessions for the branching index character, despite the fact that the study was carried out on 208 accessions belonging to nine different species of Amaranthus (including A. quitensis) from the USDA (United States Department of Agriculture). In our study, 138 of the 139 accessions presented branches all along the stem, which is common in wild, weedy o semi-cultivated Amaranthus species, such as A. quitensis, compared to the single stem observed in typically cultivated species, such as A. caudatus, A. cruentus and A. hypochondriacus (Ruiz Hernández et al., 2013; Thapa & Blair, 2018). Similar to our results, most of the native amaranth accessions collected from different parts of the Philippines had all branches along the stem (Gueco et al., 2016). However, Tapia et al. (2017) did find that the stem branching index was one of the most discriminating characters between groups of accessions conserved in the INIAP Germplasm Bank. These last results may be due to the fact that they studied more than 400 accessions be longing to different species and with very diverse origins, from native samples collected in the Ecuadorian highlands to samples from international germplasm exchanges, essentially of cultivated species. Similarly, Gerrano et al. (2017) reported high levels of diversity for branching index in thirty-two Amaranthus species collected from different regions in the world and conserved in the genebank of the Agricultural Research Council, South Africa.
The descriptors related to pigmentation of stem (D15), leaf (D18) and inflorescence (27) were significantly discriminant between accessions from different provinces, both for those collected in the 1980s and for those collected more recently (Table 6). Thapa & Blair (2018) found significant differences in stem colour, leaf pigmentation and inflorescence colour across different clusters of Amaranthus accessions. Likewise, these three qualitative characters were also polymorphic and showed high diversity levels in Amaranthus species conserved in the genebank of Pretoria, South Africa (Gerrano et al., 2017) or in the genebank of INIFAP, México (Ruiz Hernández et al., 2013), and in amaranth accessions collected from different districts of Malawi (Nyasulu et al., 2021).
Table 5 Total and by province (Imbabura, Tungurahua, and Cañar) frequencies for the polymorphic qualitative traits in the 50 ataco (A. quitensis) accessions collected between 1981 and 1986 (Collect A; five descriptors) and in the 89 accessions collected in 2014 and 2015 (Collect B; seven descriptors)
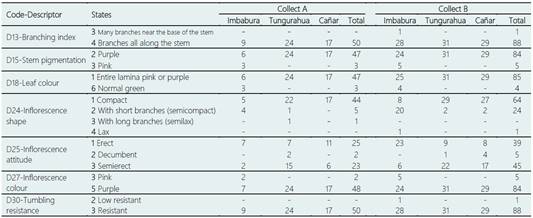
Tapia et al. (2017) also found that one of the most discriminating characters between groups of Amaranthus accessions conserved in the genebank of INIAP-Ecuador was the colour of the inflorescence. In our study, we found that around 95% of the accessions presented a more intense pigmentation (purple) in stem, leaf and inflorescence, while only a few accessions from Imbabura, three from Collect A (A1, A2 and A7) and five from Collect B (A56, A58, A61, A68 and A79), showed a lower intensity in the pigmentation of at least two of the three structures: stem and inflorescence of pink colour, and green leaves (Table 5; see Supplementary Material 5).
Therefore, we could consider the existence of two color morphotypes in the province of Imbabura, one majority (morphotype I) with a more intense pigmentation (purple) and another minority (morphotype II) with lighter pigmentation (pink stems, and usually pink inflorescences and green leaves). The predominant morphotype I is highly appreciated by native farmers in the Andean region, since the purple inflorescences are often widely used to colour traditional beverages such as "colada morada", “horchata” or “draque” (Peralta et al., 2008; Patrimonio Alimentario, 2013). While the morphotype II has only been preserved in a few farms or chakras in the province of Imbabura, essentially for its ornamental value (Personal communication from the farmers, during the elaboration of the survey carried out in the collection of 2014 and 2015). Brenner et al. (2010) reported the existence of a wide range of pigmentation in Amaranthus species due to expression of various betacyanin pigments, and that more than six major genes have been identified that determine patterns of pigment expression. So that, it would be interesting to carry out future analysis in the accessions with different colour morphotypes to check if they show different expression of these major genes related to pigmentation.
The descriptors of the shape (D24) and attitude (D25) of the inflorescence presented the highest levels of polymorphism, having detected up to four and three different states, respectively (Table 5). Regarding the inflorescence shape, 77.7% (108/139) of the accessions showed a compact inflorescence and 20.9% (29/139) semicompact, in addition of one accession with a semilax inflorescence (A13; Tungurahua-Collect A) and another with lax inflorescence (A57; Imbabura-Collect B). Respect to the inflorescence attitude, 46% (64/139) of the accessions presented an erect inflorescence, 49% (68/139) semierect, and decumbent the remaining 5% (Table 5; see Supplementary Material 5). Both descriptors had a highly significant contribution (p < 0.0001) in the differentiation between provinces for the samples of Collect B, while in Collect A significant differences between provinces were only obtained for the character of inflorescence shape (Table 6).
Table 6 Discriminant values obtained for polymorphic qualitative descriptors in the agro-morphological differentiation of accession groups collected in different provinces for the same collection (see Collect A and Collect B) and for groups of accessions collected at different times (see Collect A vs. Collect B), using the Chi-square test (X 2) and Pearson’s (C) and Cramér’s V contingency coefficients. Significant (p < 0.05) discriminant values are indicated in bold
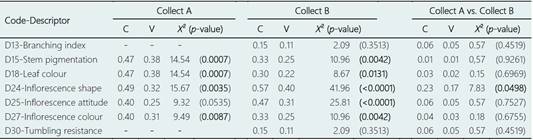
These descriptors usually present different levels of discrimination between groups of accessions according to the number and origin of the accessions under study. Tapia et al. (2017) detected that both characters were highly discriminant between groups of accessions from the INIAP Amaranthus collection, while Thapa & Blair (2018) found no significant differences between groups of accessions of different USDA amaranth species for both morphological characters. On the other hand, Gerrano et al. (2017) found that the inflorescence attitude was a monomorphic character, while the inflorescence shape presented the highest diversity index value, indicating that this trait contributed to most of the genetic diversity among the Amaranthus species conserved in the genebank of Pretoria. In our study, 77.7% of the accessions showed a compact inflorescence and 46% erect inflorescence. Accessions with these characteristics are important to take into account for future amaranth improvement programs since a compact inflorescence is usually related to a higher yield and erect inflorescences also facilitate harvesting (Tapia et al., 2017).
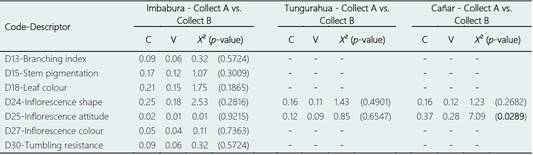
When accessions from different collects were compared without considering the separation by provinces, significant differences were only detected between both collects for the inflorescence shape character (Table 6). On the other hand, when comparing the accessions collected in the same province but in different collects, no significant differences were detected for any of the qualitative characters analyzed in the provinces of Imbabura and Tungurahua, and significant differences were only found for inflorescence attitude in the province of Cañar (Table 7).
Table 7 Discriminant values obtained for polymorphic qualitative descriptors in the agro-morphological differentiation of accession groups collected in the same province (Imbabura, Tungurahua, and Cañar) at different times (Collect A vs. Collect B), using the Chi-square test (X 2), and Pearson’s (C) and Cramér’s V contingency coefficients. Significant (p < 0.05) discriminant values are indicated in bold
The Spearman’s correlation matrices (and their corresponding p-values) between the qualitative polymorphic characters analyzed in the 50 accessions of Collect A and in the 89 accessions of Collect B, are shown in Supplementary Material 6 and 7, respectively. In both cases, only high absolute correlation values (> 0.65) and highly significant (p < 0.0001) were obtained between three characters related to pigmentation (D15-stem pigmentation, D18-leaf colour, and D27-inflorescence colour): the accessions that presented stems with purple pigmentation also showed leaves with pink or purple entire lamina and purple inflorescences, while the accessions that presented pink stems usually showed leaves with green lamina and pink inflorescences (see Supplementary Material 5). The rest of the correlations turned out to be less than 0.5 and, in general, not significant.
3.3 Cluster analysis based on phenotypic characters
The data obtained for the 11 quantitative characters and the seven polymorphic qualitative characters analyzed in the 139 ataco accessions were used to obtain a Gower’s distance matrix and a dendrogram was constructed using Ward's hierarchical clustering method showing the phenotypic relationships between these ataco accessions (Figure 2). The Mantel test revealed a good and highly significant cophenetic correlation (r = 0.533; p = 0.0001), indicating that the dendrogram provides a good fit to Gower’s distance matrix (Rohlf, 2008).
The dendrogram showed the 139 accessions divided into two major Groups (I and II) at a dissimilarity level of 5.53 (Figure 2). Group I contained 58 accessions, all of them belonging to Collect B, while the 81 accessions included into Group II corresponding to the 50 accessions of Collect A plus the remaining 31 of Collect B. Quantitative descriptors D7 (grain yield per plant) and D11 (days to physiological maturity or to harvest) showed highly significant differences between Groups I and II (p = 0.0017 and p < 0.0001, respectively; see Supplementary Material 8). Regarding the qualitative descriptors, it was noticed that the highly significant differences were detected between both groups of accessions for descriptor D25 (inflorescence attitude, p = 0.0092; see Supplementary Material 9). The accessions of Group I were characterized by being more precocious, all of them had 168 days to physiological maturity (see Figure 2 and Supplementary Material 2), and a grain yield per plant mean value of 21.6 g; while the accessions of Group II had a later maturity, they presented a mean of 175.6 days to physiological maturity (77 of the 81 accessions showed values of 176 days; see Figure 2 and Supplementary Material 2), in addition to a lower mean value of grain yield per plant (17.2 g; see Supplementary Material 8). Regarding the qualitative character of inflorescence attitude, 64% (37/58) of the accessions of Group I presented semierect inflorescences and 31% (18/58) were erect ones; while in Group II the accessions with erect inflorescences (57%; 46/81) predominate compared to the semierect ones (38%; 31/81) (see Figure 2 and Supplementary Material 2).
Group I showed two Subgroups (I.1 and I.2) differentiated at 2.40 dissimilarity level (Figure 2). Subgroup I.1 included 19 accessions, while Subgroup I.2 contained 39 accessions. In both cases we found accessions collected in the three provinces under study. It was found that up to 7 of the 11 quantitative descriptors analyzed showed significant differences between the Subgroups I.1 and I.2, so that the accessions of cluster I.1 presented significantly higher mean values for plant height, inflorescence length and seed size, while accessions from cluster I.2 showed significantly higher mean values for petiole length, leaf length and width, and grain yield per plant (see Supplementary Material 8). For qualitative descriptors, again the inflorescence attitude was the only one that presented highly significant differences between both subgroups (p < 0.0001; see Supplementary Material 9). Thus, 95% (18/19) of the accessions of Subgroup I.1 presented erect inflorescences, while in Subgroup I.2 92% (36/39) of the accessions showed semierect inflorescences (see Figure 2 and Supplementary Material 2).
On the other hand, in Group II were defined three Subgroups (II.1, II.2 and II.3), the Subgroup II.1 was separated from the other two at 3.50 dissimilarity level, and the clusters II.2 and II.3 at a dissimilarity level of 3.12 each other (Figure 2). Thirty-one accessions were found in Subgroup II.1 and 42 in Subgroup II.2, and in both cases, there were accessions from the three provinces and from the two collects. Subgroup II.3 only contained eight accessions, all of them from the province of Imbabura, three from Collect A (A1, A2 and A7) and five from Collect B (A56, A58, A61, A68 and A79) (Figure 2).
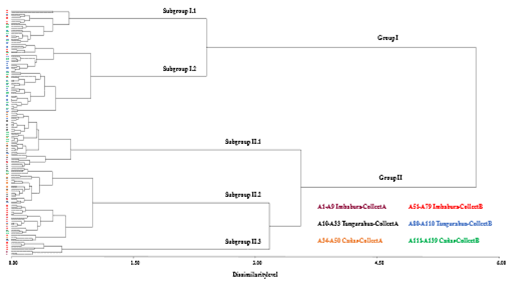
Figure 2 Ward’s hierarchical clustering dendrogram showing the phenotypic relationships among the 139 ataco accessions (A1 to A139) collected in the provinces of Imbabura, Tungurahua, and Cañar on 1981-1986 (Collect A) and 2014-2015 (Collect B). It is based on data from eleven quantitative and seven qualitative traits using the Gower’s distance.
Likewise, with respect to the 50 accessions of Collect A, it was noticed that approximately 50% (23/50) were located into Subgroup II.1 and about the remaining 50% (24/50) were included into Subgroup II.2 (Figure 2).
Significant differences were detected between Subgroups II.1, II.2 and II.3 for eight of the 11 quantitative descriptors studied (see Supplementary Material 8). These differences are due to the divergence between the accessions of Subgroup II.3 with respect to those of the other two subgroups, which in turn did not present significant differences between them for any of the 11 quantitative characters (see Supplementary Material 8). Thus, the accessions of Subgroup II.3 compared to those of Subgroups II.1 and II.2 showed significantly lower mean values for plant height (159.13 cm compared to mean values > 174 cm in Subgroups II.1 and II.2), days to flowering (82.75 days vs. mean values > 90 days in II.1 and II.2), and days to physiological maturity (172 vs. 176 days in II.1 and II.2).
In addition, the accessions of Subgroup II.3 presented mean values significantly higher than those of the accessions of Subgroups II.1 and II.2 for the characters length and width of the leaf, and grain yield per plant (24.11 g vs. 17.08 and 15,96 g in II.1 and II.2, respectively) (see Supplementary Material 8). Regarding the qualitative descriptors, highly significant differences (p < 0.0001) were detected, on the one hand, for inflorescence attitude of Subgroup II.1 with respect to the other two subgroups and, on the other hand, for the three descriptors related to pigmentation (of stem, leaf and inflorescence) in the case of Subgroup II.3 vs. Subgroups II.1 and II.2 (see Supplementary Material 9). For inflorescence attitude, 100% (31/31) of the accessions of Subgroup II.1 presented semierect inflorescences, while 90% (38/42) of the accessions of Subgroup II.2 and 100% (8/8) of those of Subgroup II.3 showed erect inflorescences. In addition, the eight accessions of Subgroup II.3 were the only ones that presented a less intense pigmentation in stem, leaves and/or inflorescence with respect to all the other acces sions studied, so they constitute a colour morphotype different from the typical morphotype of more intense coloration observed in the rest of the accessions (see Table 5).
Regarding the phenotypic relationships established in the dendrogram, in general, the accessions do not seem to be clustered according to their geographical origin, since, except in Subgroup II.3, in the rest of the subgroups we find mixed accessions from the three provinces and from both collects. Similarly, Nyasulu et al. (2021) also found no association between amaranth accessions and their geographical origin from different districts of Malawi.
Most of the quantitative characters used to construct the dendrogram turned out to be significantly discriminant between the groups and subgroups of accessions ob tained, while among the qualitative ones, only the three descriptors related to pigmentation and, especially, the inflorescence attitude discriminating between different clusters. Therefore, the different groups of accessions defined in the dendrogram showed differential character istics with respect to traits of great agronomic importance, such as plant height, inflorescence length, seed size, grain yield per plant, days to physiological maturity and inflorescence attitude, which are usually of great interest to plant breeders (Gerrano et al., 2015; Tapia et al., 2017).
Cluster analysis has proven to be an effective method for grouping germplasm with common agro-morphological characteristics. In general, the dendrogram showed a significant level of phenotypic diversity (especially with regard to quantitative characters) in the ataco germplasm, between and within groups of accessions. Therefore, this agro-morphological characterization provides preliminary information that could serve to enhance the use of this plant genetic resource in future breeding programs, as well as to carry out more efficient management of the black amaranth collection from INIAP Germplasm Bank.
4. Conclusions
The diversity detected for the quantitative agro-morphological characters analyzed in accessions from different collects does not seem to have undergone significant changes in the last 35 years, which would indicate that farmers in the Andean region are carrying out adequate on-farm conservation of the native black amaranth germplasm. However, while in the accessions collected in the early 1980s practically no significant differences were detected between provinces, in the accessions collected recently significant heterogeneity was found between the three provinces under study.
In the same province, the differences detected between the accessions collected at different times, indicate that, except for the character days to physiological maturity (days to harvest), for which in the three provinces the most recently collected accessions have turned out to be more precocious, significant differences have been detected for different quantitative characters according to the province, and this could be related to the management of the crop and the traditional uses of the ataco in each of them.
With respect to the qualitative characters analyzed, significant contributions were found of stem pigmentation, leaf colour, inflorescence colour, inflorescence shape and inflorescence attitude on differentiated accessions from different provinces.
Agro-morphological analysis can be very useful to characterize Amaranthus germplasm. The quantitative and qualitative characters used in the current study revealed a significant level of phenotypic diversity in the ataco landraces collected in Andean region and conserved at INIAP genebank. The diversity detected for some characters may be of great interest to use the black amaranth for future Amaranthus breeding programs in Ecuador.