1. Introduction
The Cucurbitaceae is an important plant family worldwide, comprising more than 120 genera (Teppner, 2004). In Peru, the Cucurbitaceae family comprises 12 genera and 104 species, with 26 endemic species (Brako & Zarucchi, 1993). The Cucurbita genus includes three economically important species: C. moschata (Duchesne ex Lam.) Duchesne ex Poir. 1786, C. maxima Duchesne, 1786, and C. pepo L., 1753 (Paris & Brown, 2005). The economic importance of the species of the Cucurbitaceae family relies not only on the food and nutritional field but also on the medicinal field. These species are rich in terpenoids, alkaloids, steroids, and others, responsible for various therapeutic effects such as antihyperglycemic, anticancer, antimicrobial, anti-inflammatory and immunomodulatory activities (Mukherjee et al., 2022). Likewise, different parts of the Cucurbita species have significant therapeutic effects against several diseases, showing antimicrobial, antiviral, antidiabetic, anticancer and other activities (Malviya & Sharma, 2021).
The ‘loche’ (C. moschata), a unique pre-Columbian squash locally grown in north coastal Peru, has special agronomic and culinary characteristics (Ugás & Bustamante, 2006). The ‘chuyán’ is another cultivated variety of C. moschata that grows in the Andes mountains of Lambayeque and Cajamarca (Peru) (Delgado-Paredes et al., 2014). The ‘chisguín’ (C. ecuadorensis Cutler & Whitaker, 1968) is a semi-cultivated species, endemic to Ecuador, occasionally found in the Lambayeque Andes at altitudes above 2000 meters above sea level. The fruits can be considered as a nutritional supplement due to the presence of carbohydrates, lipids and proteins, with a natural antioxidant potential, although all the extracts evaluated did not show antibacterial activity (Castillo & Moncayo, 2019).
An efficient plant propagation system is, therefore, essential for mass propagation, eventual transformation, and in vitro breeding through a selection of somaclonal variation and somatic hybridization. Shoot regeneration on the relationship between organogenic capacity and endogenous hormonal contents in pumpkin (C. moschata) was reported by Zhang et al. (2008). In another study, cotyledon explants of sweetgourd (C. moschata) were cultured on MS culture medium supplemented with different plant growth regulators reaching a high shooting frequency (Kabir et al., 2016). Also, the efficiency of haploidization in C. maxima and C. moschata through another culture was evaluated, reaching up to 47.3% of haploid plants (n=20) (Kurtar et al., 2016). An extensive review of advances in tissue culture of cucurbits has been recently published (Dhumal et al., 2020).
In recent years, the literature does not report studies on micropropagation of C. moschata and C. ecuadorensis. However, several studies have been carried out on micropropagation and somatic embryogenesis in other Cucurbita species such as pumpkin (C. pepo), using shoot tips, cotyledon and hypocotyl segments as explants (Ananthakrishnan et al., 2003; Stipp et al., 2012). In another species of pumpkin (C. maxima) and ash gourd (Benincasa hispida Thunb. Cogn., 1881) (Haque et al., 2008) micropropagation was also carried out successfully using shoot tips and nodal segments.
On the contrary, the literature is abundant in studies carried out on C. moschata for the antioxidant capacity of the fruit pulps (Miljić et al., 2021); the pro-osteogenic properties of leaf extracts evidenced from in vitro human primary cell cultures (Lambertini et al., 2021); the in vitro effects of seed extracts on echinococcus granulosus protoscoleces, a parasite that causes a zoonotic disease (Hesari et al., 2020), and the antibacterial activity of total extract from leaves against clinical strains of Staphylococcus aureus Rosenbach, 1884 and Escherichia coli (Escherich, 1885) (del Castillo et al., 2017), among other studies. Despite all these studies, there is a scientific gap in the micropropagation of species of the genus Cucurbita, so this study aims to contribute to this knowledge. Therefore, the objective of this study was to establish an efficient clonal propagation protocol of ‘loche’ (C. moschata), using apical buds and nodal segments in ex vitro, in vitro semi-aseptic and in vitro aseptic conditions, as well as the in vitro clonal propagation of ‘chuyán’ (C. moschata) and ‘chisguín’ (C. ecuadorensis).
2. Materials and methods
Collection places
The collections of stem cuttings and fruits of ‘loche’ (Cucurbita moschata) were carried out in the locality of Mórrope (Lambayeque), on the northern coast of Peru, and the fruits of ‘chuyán’ (C. moschata) and ‘chisguín’ (C. ecuadorensis) in the localities of Cutervo (Cajamarca) and Kerguer (Lambayeque), respectively; both localities located in the Andean region between 1500 to 2500 meters above sea level (Figure 1).
Plant material
The ‘loche’ (C. moschata) produces fruits with seeds, generally sterile, and fruits without seeds (Figures 2a, 2b), which es why the plant material used was stem cuttings (Figure 2c), with 50-70 cm long and 10-15 nodal segments, excised from adult plants, after the second harvest of fruits and in the best physiological and phytosanitary conditions. This plant material was established under greenhouse conditions, in a sterilized soil:sand (1:1) substrate. The environmental conditions of the greenhouse were: temperature of 24 ± 2 oC and natural lighting. The cuttings received continuous irrigation.
In the case of ‘chuyán’ (C. moschata) (Figure 2d) and ‘chisguín’ (C. ecuadorensis) (Figure 2e), seeds were obtained from ripe fruits.

Figure 2 Propagation units. a. ‘Loche’ fruits, b. ‘Loche’ fruits with infertile seeds and fruits without seeds. c. ‘Loche’ cuttings. d. ‘Chuyan’ fruits (the yellow fruit in the center is ‘chisguín’) and e. ‘Chisguin’ fruits.
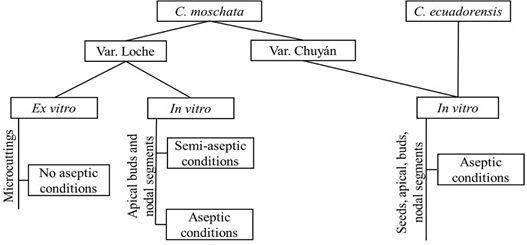
Figure 3 General diagram of the procedure used in the ex vitro and in vitro micropropagation of Cucurbita moschata and C. ecuadorensis.
Disinfestation of samples
In the laboratory stem cuttings of ‘loche’ were initially treated with the insecticide 0.25 cc/L Absolute 60 SC (Spineloram 60 g/L-1). Subsequently, in the disinfestation process of apical buds and nodal segments, under ex vitro conditions, running water with detergent was used. In in vitro semiseptic conditions running water, detergent and 0.2% benomyl (Benlate) solution for 60 min were also used, and in in vitro disinfestation process several treatments were tested using 70% ethanol for one min, as the first disinfestant, and 5.25% NaClO for 3 or 10 min, with Tween 20 (2-3 drops), or HgCl2 0.5% for 3 min, as a second disinfestant. Likewise, the antibiotics rifampicin 30 mg/L-1 or rifampicin 30 mg/L-1 plus chloramphenicol 50 mg/L-1 were supplemented for 24 h.
In the disinfestation of ‘loche’, ‘chuyán’ and ‘chisguín’ seeds, only 70% ethanol were used for five minutes and 5.25% NaClO for 15 minutes. The disinfestants were removed with sterilized distilled water three consecutive times.
Ex vitro culture of apical buds and nodal segments of ‘loche’
Apical buds and nodal segments 5 cm long with lateral buds of 1-3 cm height and visible root primordia were used. If present, the leaf, the tendril caulinar and the floral bud were removed with a scalpel blade. The explants were grown on two types of substrates: sterilized soil and polyethylene foam sheet, while the irrigations were continuous with the MS mineral solution (Murashige & Skoog, 1962) and MS supplemented with 2.0 mg/L-1 IBA (indole-3-butyric acid). In this experiment, 100 explants were evaluated, between apical buds and nodal segments, per each treatment. Evaluations were made after 30 days.
In vitro culture in semi-aseptic conditions of apical buds and nodal segments of ‘loche’
The same types of explants (apical buds and nodal segmentes), with the same morphological characteristics, were used in the establishment of these treatments. The explants were placed on several culture medium containing MS salts with IAA (indole-3-acetic acid) (0.5 and 1.0 mg/L-1) or NAA (0.5 and 1.0 mg/L-1) or BAP (0.5 and 1.0 mg/L-1) or MS supplemented with 0.25% activated charcoal. In this experiment 50 culture vessels (150x25 mm) were inoculated, between apical buds and nodal segments, for each treatment and the experiment was repeated twice. Evaluations were made after 60 days.
In vitro culture in aseptic conditions of apical buds and nodal segments of ‘loche’
Explants of ‘loche’ collected from plants established in the greenhouse were used in this experiment. Apical buds and nodal segments were also used in the establishment of these treatments. Additionally, explants were surface sterilized by immersion with commercial bleach containing 5.25% sodium hypochlorite (NaOCl) for 2 min followed by three washings with sterilized distilled water and 30 mg/L-1 rifampicin for 24 h. After disinfestation, the explants were placed on several culture medium containing several concentrations of auxins (IAA and NAA), citokinins (BAP) and gibberellin (GA3) with IAA (0.5 and 1.0 mg/L-1), NAA (0.5 and 1.0 mg/L-1) or BAP (0.5 and 1.0 mg/L-1) or MS supplemented with 0.25% activated charcoal. In this experiment 25 culture vessels (150x25 mm) were inoculated, between apical buds and nodal segments, for each treatment and the experiment was repeated twice. Evaluations were made after 60 days.
In vitro culture in aseptic conditions of seeds, apical buds and nodal segments of ‘loche’, ‘chuyán’ and ‘chisguín’
Aseptic seeds were cultivated in MS culture medium and once germinated, apical buds and nodal segments of seedlings were cultivated in the culture medium treatments formulated for elongation and rooting. In ‘chuyán’ and ‘chisguín’, 25 explants were used, between apical buds and nodal segments, for each treatment and the experiment was repeated twice. Since ‘loche’ rarely forms sexual seed and a sufficient number of explants could not be obtained, the experiment was not repeated. Evaluations were made after 60 days.
Figure 3 summarizes the procedure followed in the ex vitro and in vitro micropropagation of C. moschata and C. ecuadorensis.
Composition of the culture medium and environmental incubation conditions
All the culture media incorporated the mineral salts MS supplemented with the vitamins 100 mg L-1 m-inositol and 1.0 mg/L-1 thiamine.HCl, 2.0% sucrose and various growth regulators, depending on the species and the phase of the in vitro culture.
In all experiments, the pH of the culture medium was adjusted to 5.8±0.1 with 1N NaOH and 1N HCl using electronic pH indicator. Only in in vitro experiments under aseptic conditions, the culture medium was solidified with 0.6% agar-agar before autoclaving at 121 oC and pressure of 15 lbs/psi for 20 min. The material was incubated a temperature of 26±2 oC under a 16-h photoperiod (70 µmol m-2s-1). The data were subjected to statistical analyses.
Preparation of histological samples
Nodal segments with root primordia were used in the preparation of the histological samples. The cuts were prepared using a razor blade and observed to the natural without the application of any type of coloring.
Statistical analysis
The data was subject to analysis of variance, using a Tukey’s multiple range test at a significance level of 95%. Statistical software SPSS version 15.0 and Microsoft Word, Excel 2013 and Megastat programs were also used.
3. Results and discussion
Disinfestation of ‘loche’ explants for in vitro cultures
In the disinfestation of apical buds and nodal segments of ‘loche’ several treatments were tested but only in the protocol where 5.25% NaOCl was used for three min and 30 mg/L-1 rifampicin for 24 h, the contamination rate was 0.0% and the survival rate was 100%, after 45 days of culture. In other treatments, the contamination rate ranged from 20 to 100% and even the browning rate between 40 to 100%, processes that led to the death of the explant, after 45 days of culture.
The high rate of contamination and the death of stem tips and nodal segments are attributed to the presence of the ‘green worm’ (Diaphania hyalinata L., Lepidoptera: Pyralidae), although the explants were previously treated with 0.25 cc/L Absolute 60 SC (60 g/L-1 Spineloram). The insect larvae deteriorate and contaminate the internal tissues of the explant with bacteria and fungi.
In the process of disinfestation of nodal segments of pumpkin (Cucurbita maxima) and ash gourd (Benincasa hispida), 1% savlon (v/v) and two drops of Tween-80 were used for 5 min and different concentration of HgCl2 at different periods of time; after 7 days of incubation, 80% of the explants were free from contamination and tissue killing when pumpkin explants with 0.03% HgCl2 at 3.0 min and ash gourd explants with 0.03% HgCl2 at 3.5 min (Haque et al., 2008). In our study, the use of 0.5% HgCl2 for 3.0 min in the disinfestation of ‘loche’ caused the death of the tissues.
Ex vitro culture of apical buds and nodal segments (leaf-bud microcuttings) of ‘loche’
In the ex vitro propagation of apical buds and nodal segments of ‘loche’, the polyurethane foam substrate (80 to 100% rooting) was better than the sterilized farmland substrate (60 to 80% of rooting), attributing the 20% increase in rooting, for each type of substrate, to the supplement of 2.0 mg/L-1 IBA (Table 1, Figure 4a). Shoots elongation and survival rate, in each type of substrate, was 100%; however, after 30 days of culture, the rooted plantlets died (Figure 4b).
Table 1 Treatments tested in the ex vitro propagation of apical buds and nodal segments of ‘loche’ (C. moschata), after 30 days of culture

Average of 100 explants/treatment. MS (Murashige and Skoog mineral salts) and IBA.
Values indicated by different letters show significant differences at p < 0.05 (Tukey’s multiple range test).
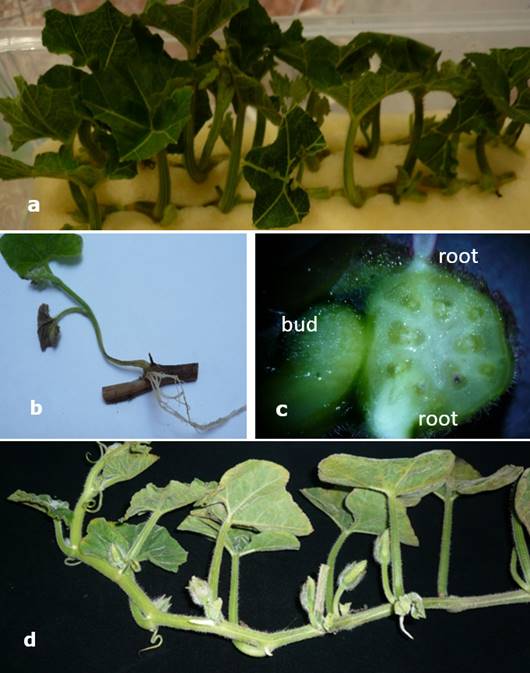
Figure 4 Cucurbita moschata (‘loche’) propagation. a. Cuttings on polyurethane foam substrate, b. Dead cutting, c. Histological section showing that the bud has no vascular connection with the root and d. Cutting 80 cm long used as a clonal propagation unit.
The ‘leaf-bud cuttings’ or ‘micro-cuttings technique’ was developed as a novel method for rapid propagation of cassava (Chant & Marden, 1958). Later, the method was developed and improved by Pateña & Barba (1979) at the University of the Philippines. The modifications tested at the International Center for Tropical Agriculture (CIAT) made the method more efficient and less expensive (Toro et al., 1982). Recently using the leaf bud technique for rapid propagation of cassava it would be possible to reach an annual multiplication rate of 1:72 over the traditional methods using mature stem cuttings from 12-month-old plants (around 1:5) (Neves et al., 2020).
In other species such as Bauhinia racemosa, a leguminous tree with medicinal importance, methods such as in vitro propagation and ex vitro rooting using nodal explants obtained from sexually mature tree (about 20 years old) have been combined (Sharma et al., 2017). In Pseudostellaria heterophylla, an important Chinese medicinal plant, protocols for in vitro regeneration and ex vitro rooting using nodal segments of greenhouse plants were developed (Wang et al., 2020). An extensive review of various vegetative propagation methods to improve plant growth has been reported by Awotedu et al. (2021). In the study, the technique developed in cassava has been adapted to the ex vitro propagation of ‘loche’, it would be necessary to improve the development of the root system so that the method achieves greater efficiency.
Histological observations demonstrated the formation of 2-3 roots in the nodal segments. However, these roots did not present a vascular connection with the bud in formation, and the death of the bud was attributed to this characteristic (Figure 4c). This is the reason that in field conditions, farmers use cuttings with a length of 50 to 70 cm (Figure 4d), burying about 2/3 of the cutting, ensuring that the root-shoot vascular connection of some nodal segments guarantees the flow of water and nutrients to the new plant.
In vitro culture of apical buds and nodal segments of ‘loche’ under semi-aseptic conditions
The results in the propagation of apical buds and nodal segments of ‘loche’ in semi-aseptic condi tions are shown in Table 2, observing the highest rate of bud elongation (75 and 80%) in the treat ments with MS mineral salts supplemented with 0.5 and 1.0 mg/L-1 BAP. Treatments with MS mineral salts, supplemented with 0.5 and 1.0 mg/L-1 IAA, in duced the highest rooting rates, slightly exceeding treatments with 0.5 and 1.0 mg/L-1 NAA. However, in both treatments with auxins, the rooting rate did not exceed 50%. In all the treatments tested, with BAP, IAA and NAA, the contamination rate with fungi and bacteria was 100%. However, in additional treatment with MS mineral salts supplemented with 0.25% activated charcoal (AC), the contamination rate was reduced to 20% while the shoot elongation and rooting was 60% and 40%, respectively. The plantlets were transferred to greenhouse conditions and then to the definitive field with a 50% survival rate (Figure 5a).

Figure 5 a. Acclimatization of plantlets of ‘loche’ (C. moschata), b. Plantlets of ‘loche’ (C. moschata), obtained from apical bud of greenhouse plants (left); ‘chuyán’ (C. moschata) (middle) and ‘chisguín’ (C. ecuadorensis), obtained from seed (right), after 120 days of culture. The growth and development of the in vitro plantlets were very similar in the three genotypes studied.
Table 2 Effect of growth regulators and activated charcoal (AC) on in vitro shoot elongation and rooting of apical buds and nodal segments of ‘loche’ (C. moschata) under semi-aseptic conditions, after 60 days of culture
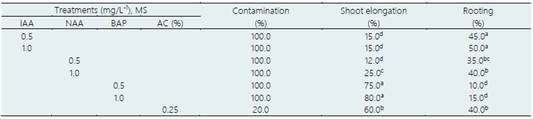
Average of 50 explants/treatment. Murashige and Skoog mineral salts without sucrose and vitamins.
Values indicated by different letters show significant differences at p < 0.05 (Tukey’s multiple range test).
A pioneering study, under semi-aseptic sterilization conditions, was carried out on numerous Begonia lines using non-sterilized explants of leaves (about 2.0 cm2 in area), grown onto half-strength Knop's mineral salts medium and solidified with 1% agar, although with limited success and without reporting contamination rates (Bowes, 1990). In Vallisneria americana, a submerged and perennial hydrophyte distributed from North America to Central America, five experiments were carried out with stages of pre-sterilization, sterilization, and post-sterilization of explants and culture in monophasic or biphasic nutrient media; however, the results expressed in the regeneration-contamination balance were somewhat discreet (Ruiz-Carrera & Sánchez, 2008). In our study, although in most of the treatments tested the contamination rate reached 100%, the shoot elongation and rooting levels made it possible to establish plantlets in the field with 50% survival. Likewise, the high rate of contamination observed indicated that the tested antibiotics had no effect on contamination control, which is why it is important to test the latest generation of antibiotics.
In vitro culture of apical buds and nodal segments of ‘loche’, obtained from greenhouse plants, under aseptic conditions
The results of the effect of the auxins IAA and NAA at low concentrations and various auxin-BAP-GA3 interactions, in the morphogenic responses of nodal segments obtained from greenhouse plants of ‘loche’ are shown in Table 3. Only in the 0.02 mg/L-1 IAA or NAA interactions with 0.5 mg/L-1 BAP and 0.2 mg/L-1 GA3, 50% shoot elongation, 100.0% rooting and 25% survival were achieved (Figure 5b).
The effect of the IAA-GA3 and IAA-BAP-GA3 interaction on the morphogenic responses of apical buds and nodal segments of in vitro seedlings of ‘loche’ is shown in Table 4. In all the treatments tested, shoot elongation was 70 to 100%, although the rooting rate (90%) and survival (100%) were higher in the treatment with 0.02 mg/L-1 IAA and 0.02 mg/L-1 GA3. This formulation, named Piper's culture medium, has been widely used in the micropropagation of numerous species of ‘matico’ (Piper spp.) (Delgado-Paredes et al., 2012) and some tree species of the seasonally dry tropical forest of the north of Peru (Delgado-Paredes et al., 2021). This formulation offers the advantage that it simultaneously induces shoot elongation and rooting without the need to use two culture medium formulations, which leads to significant cost and time savings.
After 120 days of culture, the plantlets obtained in the various treatments indicated in Table 3 and Table 4 presented physiological disorders such as apical necrosis and hyperhydricity (vitrification).
In vitro seeds germination of ‘chuyán’ and ‘chisguín’, after 15 days of culture
Seed germination was higher in ‘chuyán’ (C. moschata) with 82.1%, while in ‘chisguín’ (C. ecuadorensis) it was 65.0%. However, seed contamination (7.2%) was observed only in ‘chuyán’, after 15 days of culture (Table 5).
Table 3 Effect of the auxins IAA and NAA, BAP and GA3 in the morphogenic responses induction, from nodal segments obtained from greenhouse plants of ‘loche’ (C. moschata), under aseptic conditions, after 60 days of culture
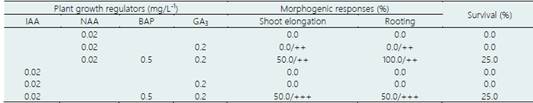
Average of 25 explantes/treatment. Shoot elongation: +, (< 1.0 cm); ++ (1.0-3.0 cm); +++ (> 3.0 cm). Rooting: -, no roots formation; +, (1); ++ (2-4); +++ (> 5 roots/explant).
Table 4 Effect of the auxins IAA, BAP and GA3 in the morphogenic responses induction, from apical buds and nodal segments of in vitro seedlings of ‘loche’ (C. moschata), under aseptic conditions, after 60 days of culture
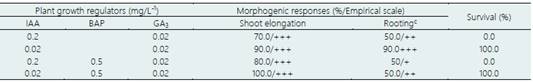
Average of 25 explants/treatment. Shoot elongation: +++ (> 3.0 cm). Rooting: -, no roots formed; +, (1); ++ (2-4); +++ (> 5 roots/explant).
Table 5 In vitro seeds germination of ‘chuyán’ (C. moschata) and ‘chisguín’ (C. ecuadorensis), after 15 days of culture

Average of 50 seeds/species. MS, 2.0% sucrose, vitamins and 0.7% agar-agar. Values indicated by different letters show significant differences at p < 0.05 (Tukey’s multiple range test).
Table 6 In vitro shoot elongation and rooting of ‘chuyán’ (C. moschata), after 60 days of culture
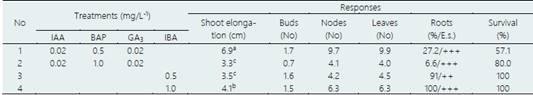
Average of 25 explants/treatment. Rooting: E.s. (empirical scale): -, no roots formed; +, (1); ++ (2-4); +++ (> 5 roots). Values indicated by different letters show significant differences at p<0.05 (Tukey’s multiple range test).
Table 7 In vitro shoot elongation and rooting of ‘chisguín’ (C. ecuadorensis), after 60 days of culture
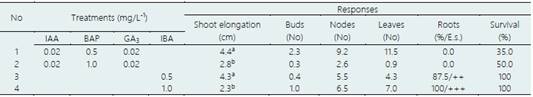
Average of 25 explants/treatment. Rooting: E.s. (empirical scale): -, no roots formed; +, (1); ++ (2-4); +++ (> 5 roots/explant). Values indicated by different letters show significant differences at p < 0.05 (Tukey’s multiple range test).
In vitro shoots elongation and rooting of ‘chuyán’ and ‘chisguín’
For both species, the shoot elongation and the number of nodes and leaves formed was greater in the T1 treatment (MS, 0.02 mg/L-1 IAA, 0.5 mg/L-1 BAP and 0.02 mg/L-1 GA3), after 60 days of culture (Table 6 and Table 7). However, the survival rate for both species was higher in the T2 treatment (MS, 0.02 mg/L-1 IAA, 1.0 mg/L-1 BAP and 0.02 mg/L-1 GA3), with 80.0 and 50.0%, respectively. The number of nodes and leaves formed was similar in both species, although much higher in treatment T1 compared to treatment T2. For both species the shoot elongation and nodes and leaves formed were similar, in both treatments (T3 and T4) although slightly higher in treatment T4, while the rooting was 100% in treatment T4, after 60 days of culture (Table 6 and Table 7). The survival rate was 100%, in both species and in the two treatments tested. At 120 days of culture, the shoot elongation was greater than 5.0 cm (Figure 5b). Physiological disorders such as apical necrosis and hyperhydricity (vitrification) were also observed.
In vitro propagation of Cucurbitaceae species has been carried out using two methods: (a) propagation with shoot tips and cotyledonal nodes obtained from seedlings and (b) other morphogenic processes such as organogenesis. However, there are few studies carried out on Cucurbita species, as evidenced by a recent review on tissue culture of cucurbits (Dhumal et al., 2020) where no more than five studies were carried out on C. maxima, C. pepo and C. ficifolia Bouché, 1837. Table 8 shows a list of species of the Cucurbitaceae family micropropagated in the last twelve years. This table shows that in the micropropagation of various species of Cucurbitaceae the best results were achieved using the MS culture medium supplemented with cytokinin BA or BAP (0.45 to 3.0 mg/L-1) alone or in combination with auxin IAA, in the same way as in our study.
For all this, it was important to carry out this study on the genotypes of C. moschata ‘loche’, of commercial vegetative propagation because it produces very little sexual seed, mostly infertile, and ‘chuyán’, a genotype widely consumed in the highlands of Peru, as well as C. ecuadorensis, a nearly threatened wild species (Santiana & Tye, 2017, León-Yánez et al., 2019) whose nutritional and medicinal importance is unknown.
Table 8 Species of the Cucurbitaceae family micropropagated in the last twelve years
| Plant species | Plant origin | Explant | Culture medium (mg/L-1) | References |
| Zehneria platysperma | Thailand | First and second nodes from seedlings | MS and BA (1.0) | Chuengpanya et al. (2020) |
| Citrullus colocynthis | Mediterranean Basin and Asia, Turkey and Nubia | Nodes from seedlings | MS, BAP (2.0) and NAA (1.0) | Tariq et al. (2020) |
| Corallocarpus epigaeus | Africa, Pakistan, Arabia and others countries | Arisen tender shoots from tubers | MS, TDZ (1.5) and IAA (1.5) | Vemula et al. (2020) |
| Cucurbita pepo | Nigerian | Cotyledonary nodes | MS and BAP (0.45) | Aworunse et al. (2019) |
| Momordica dioica | India and some parts in South Asia | Nodal explant of wild female | MS, BAP (0.5) and IAA (0.1) | Choudhary et al. (2017) |
| Ibervillea sonorae | Nortwestern Mexico | Internode segments | B5, NAA (0.5), BA (0.5) and IAA (1.0) | Arciniega-Carreón et al. (2017) |
| C. pepo | Nigerian | Cotyledonary nodes | MS | Obembe et al. (2017) |
| C. moshata | Central America ot northern South America | Cotyledon segments (Direct organogenesis) | MS and BAP (1.0) | Kabir et al. (2016) |
| Zehneria capillacea | West and Central Africa | Nodal segments from seedlings | MS and BAP (3.0) | Agogbua and Okoli (2016) |
| Cucumis melo | Not known | Nodal segments from seedlings | MS and KIN (0.1) | Sidik et al. (2012) |
| C. ficifolia | Argentina and Peru to Mexico | Cotyledon segments (Direct organogenesis) | MS, ZEA (1.0) and IAA (0.1) | Kim et al. (2010) |
4. Conclusions
The species of cucurbits studied, 'loche' and 'chuyán' (C. moschata), are of singular importance in feeding large sectors of the population in the north coast and north highlands regions of Peru, respectively. The importance of the 'chisguín' (C. ecuadorensis) is still unknown, but it is known that it is a threatened species. Micropropagation was possible in all these species, reaching acceptable levels of elongation and rooting, which opens the possibility of carrying out other studies such as germplasm conservation, callus induction, indirect organogenesis, somaclonal variation and somatic embryogenesis. However, primary difficulties in in vitro culture such as contamination, apical necrosis, and hyperhydricity (vitrification), still need to be resolved.