1. Introduction
Flavonoids are found in natural products such as fruits and vegetables and constitute one of the largest groups of polyphenolic compounds; they exhibit a wide variety of biological activity and medicinal properties (Barfi et al., 2013; Zheng et al., 2021). Due to the interest, they arouse in human health, the development of analytical methods to detect and measure flavonoid content in various matrices is necessary (Majidi & Hadjmohammadi, 2021; Tashakkori et al., 2021), being found in high proportions in peels and seeds (Albuquerque et al., 2016; Derakhshan et al., 2018; Fu et al., 2018; Guerrero-Castillo et al., 2019). Some beneficial properties include anticancer (Kim et al., 2010; Masci et al., 2016), anti-inflammatory (da Silva Sauthier et al., 2019; Dong et al., 2019; Fidelis et al., 2020; Tang et al., 2021), antimicrobial (Al-Zoreky, 2009; Asif et al., 2016; Gosset-Erard et al., 2021; Saleem&Saeed, 2020; Warnasih et al., 2020), and antioxidant activity (Russo et al., 2016; Warnasih et al., 2020), with health benefit (Saffarzadeh-Matin & Masoudi-Khosrowshahi, 2018).
Phenolic compounds are divided into 6 subclasses: flavonols, flavones, flavanones, isoflavones, flavanols, and anthocyanins. All have a similar structure (Figure 1): they consist of 2 aromatic rings, A and B, linked to 3C atoms to give an oxygenated heterocycle as the C ring (Alara et al., 2021).
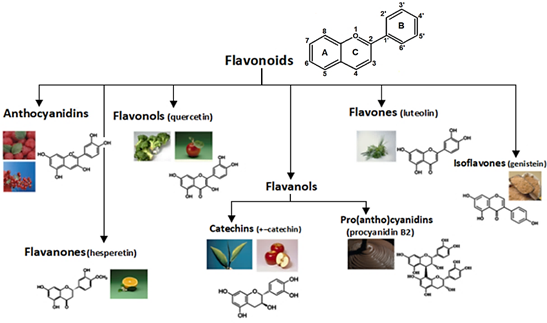
Figure 1 Basic flavonoid structure and 6 subclasses: flavonols, flavones, flavanones, isoflavones, flavanols, and anthocyanins whit similar structure. Adapted from Singh et al. (2011).
There is a growing interest in the extraction of phenolic compounds from different plant materials and their waste. Thus, the extraction, identification, and characterization of novel phenolic compounds from different materials of plant origin is a challenge due to their diversity and chemical and structural complexity (Suleria et al., 2020).
Among the phenolic compounds, flavonoids are the most predominant in almost all plants (Sembiring et al., 2018). Studies by Marina & Noriham (2014) found higher amounts of flavonoids in mango peel compared to tropical fruit peels such as papaya and guava. Ayala-Zavala et al. (2011) conducted a review highlighting the by-products of exotic fruit processing that are rich in bioactive compounds which include the importance of extraction techniques. Alara et al. (2021) reported that the amount depends on the extraction technique, considering its composition, polarity, particle size, and sample-solvent ratio. It is important to take into account the pH, pressure, and temperature for the release and stability of the compounds to be extracted (Gil-Martín et al., 2022).
Thus, the objective of this review was to make a systematic analysis in SCOPUS of scientific productions of studies on techniques for the detection and identification of flavonoids by HPLC-MS in fruit waste in Latin America. In addition, to make a review based on the methods of determination of flavonoids in Latin American fruits, 2 analyses were performed: one based on the presence of flavonoids in by-products where the pre-treatments included in the analytical method as well as the extraction and detection method is observed, and the second one only based on the method that uses the application of high-performance liquid chromatography (HPLC) with mass spectrometry (MS) detector for determination of flavonoids.
2. Techniques for the detection and identification of flavonoids in fruit waste
2.1. Different detection techniques
Little literature has been found on the use of advanced techniques applied in the identification and quantification of flavonoids in fruit waste. Scientific reviews report techniques for flavonoid analysis from two decades ago, such as paper chromatography and thin-layer chromatography, which were used in the analysis of plant extracts (Bloor, 2001; Colombo et al., 2006).
Studies have introduced the technique of high-performance liquid chromatography (HPLC) in plant matrices since 1978, according to the SCOPUS database, which improves quantification with precision and better resolution (Yoichi et al., 2006). There are also different works quantifying phenolic compounds in plant matrices and their waste, using conventional techniques based on HPLC with an Ultraviolet (UV) detector (Colombo et al., 2006; Da Silva et al., 2014).
HPLC can also be coupled to a diode-array detector (DAD), resulting in a useful tool to quantify specific polyphenols, because this technique, through its mobile phase, allows an efficient separation of compounds. The compounds are identified by comparison with the retention time (RT), so it is being widely used by researchers (Barbosa et al., 2018; Ribeiro et al., 2015).
High-performance liquid chromatography (HPLC), together with electrospray ionization (ESI) and mass spectrometry (MS), is a highly sensitive analytical tool and is the most efficient method for the characterization of both low and high molecular weight molecules, including phenolic and non-phenolic compounds (Suleria et al., 2020). It is a fast and efficient analytical method for the determination of flavonoids (da Silva Sauthier et al., 2019; Leporini et al., 2020).
2.2. Data collection
For this study, data were collected from March 2022 to January 2023, in the SCOPUS database. The search used the Boolean operators AND, OR with search criteria: ARTICLE TITLE, ABSTRACT, KEYWORDS: "fruit waste" OR "flavonoid" OR "anthocyanidins" OR "anthoxanthins" OR "flavanones" OR "flavanonols" OR "flavans" OR "Isoflavonoids" OR "flavonoids" AND ARTICLE TITLE, ABSTRACT, KEYWORDS: "HPLC-MS" OR "HPLC". Furthermore, it was delimited based on the countries of origin, study areas and by searching for original articles, the search period from 2010 to 2022 was considered. Data analysis was performed using VOSViewer software (version 1.6.18).
2.3. Data Analysis
This study was limited by keywords and Latin American country analysis for bibliometric mapping, and clustering and visualization for network analysis. To perform the systematic analysis, the strength of association was used as a measurement of similarity in a matrix that is created by normalizing the co-occurrence matrix. VOSViewer was used to perform data mining, mapping, and clustering of the retrieved articles. Keywords and countries were labeled with colored circles. The size of the circles was positively correlated with the occurrence of the keyword or countries in the title and abstract. Therefore, the label and circle size of an article was determined by its weight. The larger the weight of an element, the larger its label and circle. The manuscript describes and visualizes datasets from the bibliometric review in the R statistical software, focusing on descriptive statistics and visualizations.
3. Results
It was possible to systemize 1071 documents, where it can be observed that, for South America, most of the works were about research on fruit waste focusing on the detection of flavonoids (one or more). These are grouped into 5 countries with the highest scientific production Brazil 58.9%), Mexico (16.1%), Chile (9.2%), Argentina (7.1%) and Colombia (3.8%) and with the lower production we have 4.9% that correspond to Ecuador, Peru and others such as Uruguay, Venezuela, Paraguay and Bolivia (Figure 2a). Many of these works were carried out in collaboration with the USA or Europe. In this case, Spain is the main country accompanying this type of research with Latin American countries (Figure 2b).
In Figure 2a, the information was filtered only from original publications and the areas of greatest study correspond to Agricultural and Biological Sciences with 38.3%, followed by Chemistry and Biochemistry with 25.4% and 20% respectively.
In Figure 2b, it is possible to observe 6 clusters generated: the blue cluster where Brazil represents 58.9% of the total number of documents generated and its relationship with other countries involves mostly North American and European countries such as Canada, USA, Belgium, Spain, France, and Latin American countries, particularly Mexico. The cluster in green is led by Chile and represents 9.2% of the total number of documents. This cluster has greater Latin American collaboration with countries such as Colombia, Argentina, and Paraguay. The third cluster in red represents 16.1% of the total number of documents, with Mexico as the largest producer of information. In this group, Peru appears with very low production in topics related to the use of fruit by-products to obtain flavonoids. The other 3 clusters involve a very low production of papers in this area, and it is also necessary to note that their level and quality of relations with more experienced countries are very low.
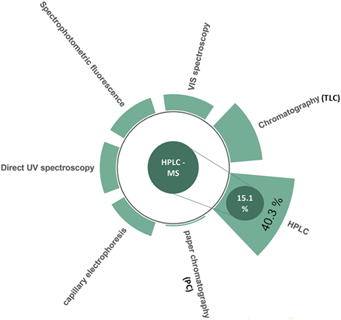
Only 15.1% of the studies evaluated used HPLC-MS (Figure 3) for the determination of these compounds. Likewise, it was observed that, when relating, the keyword HPLC-MS was linked to indicators such as quercetin, catechin, gallic acid, and anthocyanin, which are the most commonly used in this type of research.
The 40.3% correspond to the HPLC technique and 44.6% to Thin Layer Chromatography (TLC), paper Chromatography (PC), VIS Spectroscopy, Spectro-photometric fluorescence, Direct UV spectroscopy and capillary electrophoresis.
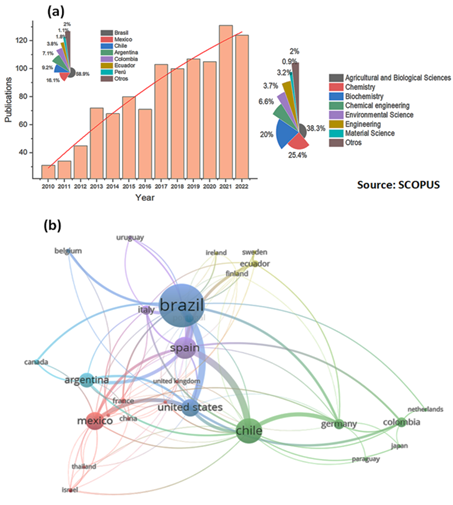
Figure 2 Number of scientific publications from 2010 to 2022 in Latin American countries on flavonoids in fruit waste (a). Network for keywords for year and countries (b). Information obtained from the database Scopus.
4. Use of fruit waste
From the systematic analysis, it was observed that waste from different fruits such as mango peels and seed kernels, banana seeds, passion fruit, poro-poro, cape gooseberries calyx, red-jambo seed and peel, lucuma seed, guaba, passion fruit peel and seed, papaya and avocado, pineapple and banana peel, pomegranate peel, peel of different citrus fruits such as grapefruit, lime, orange, among others, as well as kiwi peel and bagasse, Brazilian jaboticaba peel, and grape pomace, are considered in the fruit processing industry as waste that, if not properly used, generate environmental contami-nation. Therefore, research works have been conducted to add value to them through sustainable use. The fruits identified are shown in the word cloud in Figure 4. Considering that these materials are rich in bioactive compounds, several studies show that peels and seeds have a higher content of phenolic compounds, highlighting flavonoids for their potential benefit to human health. Studying fruit waste is important because, as manifested by Da Silva Sauthiera et al. (2019), who compare flavonoid contents present in pulp and peel of 3 mango varieties (E: Espada R: Rosa T: Tommy) from Brazil, the flavonoids analyzed in peels presented higher content than in mango pulp. Similarly, Suleria et al. (2020), in Australia, found a higher number of flavonoids in mango peel.
Regarding the pre-treatments used for subsequent extraction, the most used in waste is drying, with 60%, followed by freeze-drying with 20%. Along with this, the most used extraction methods were those using methanol in 40% as the liquid phase, followed using ethanol in 30%. The most used method for flavonoid detection is spectropho-tometry (UV-VIS) with 42.6%, followed by HPLC-DAD with 24.8% for detection (Figure 4).
The extraction of flavonoids (bioactive compounds) can be performed from fresh or dried material, provided that the drying process does not alter the composition (freeze-drying), and fine grinding to facilitate the extraction of flavonoid compounds. According to their solubility, they can be extracted with different organic solvents used in methanolic and ethanolic solutions, other subcritical water extraction techniques, and pressurized liquid extraction. Following Pattnaik et al. (2021), several factors can affect the extraction of bioactive compounds, such as flavonoids, from plant matrices and their waste or by-products, e.g., chemical properties, functionality, and end use (Pattnaik et al., 2021) are improved by using polar solvents such as methanol compared to ethyl acetate (Warnasih et al., 2020).
Flavonoids with a large number of unsubstituted hydroxyl groups or sugars are considered polar compounds, so they are moderately soluble in polar solvents such as ethanol, methanol, butanol, acetone, DMSO, and water. On the other hand, less polar aglycones such as isoflavones and flavanones tend to be more soluble in solvents such as ethers and chloroform.
As shown in the results of Figure 4, in extraction techniques, one of the most used solvents in solid-liquid extraction is methanol, something not used in Latin American research in liquid-liquid phase extraction, due to the type of waste, which is basically fruit peels and skins. This means that the treatment method must be precise for each type of waste. Normally, after this stage, spectrophoto-metric or chromatographic techniques are used.
An important technique for the determination of flavonoids is HPLC-MS. The success of this technique depends on properly following the working methodology. Identification is made using the MS detector and quantification using the area in the retention time of chromatographic peaks, which creates a unique mass spectrum for each compound. A total of 15.1% of the studies consulted in Latin America uses this method. Identification with Mass (MS) allows obtaining the list of peaks in mass-to-charge (m/z) ratio values; typical product ions are fragmented in the Mass (MS/MS). According to the studies shown in Table 1, HPCL-MS was widely used for the determination of individual flavonoid compounds.
Table 1 shows the HPLC-MS parameters that have been used to identify different flavonoids in fruit waste. There are few studies in this field in Latin America (about 11%), being surpassed by countries such as China, India, and the USA. information collected from SCOPUS database before limiting the search to Latin American countries.
Latin America is a major exporter of fresh and processed fruits, and the disposal of waste (around 15%) is a great challenge, although it can be a low-cost renewable resource for the production of functional ingredients, natural additives, and products for the pharmaceutical industry, giving added value and mitigating its environmental impact.
The use of a mass spectrometry detector allows the application of stable isotope-labeled standards, improving the quantification of flavonoids. Generating these analytical procedures designed for waste from fruit industries is very important since a suitable analytical method (e.g., for flavonoids) could be applied to certain types of food.
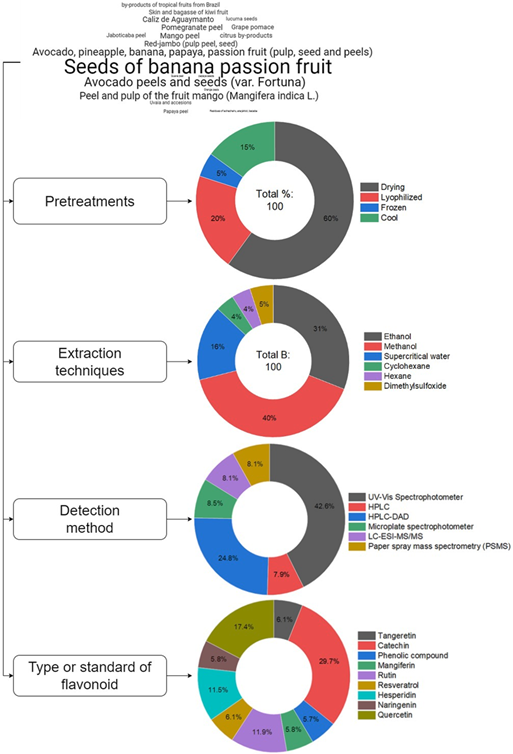
Figure 4 Word Cloud and Percentage of use of pretreatments, extraction techniques, detection methods, and types of standards used in Latin American research from 2010 to 2022.
It should be noted that very little research has been done on Latin American fruits, which does not satisfy the wide demand for studies in this field, especially if the growth of the fruit agro-export sector in Latin American countries by 2022, and the amount of research on fruit waste (Figure 5) are considered. In countries such as Peru and Chile, which have a great variety of fruits, their research is very low, and there are fruits such as blueberries, cape gooseberries, grapes, cherries, and apricots, among others.
Table 1 Application of the high-performance liquid chromatography (HPLC) method with mass spectrometry detector (MS) or MS/MS
| Waste fruits | Mobile phase (eluent) | Stationary phase | Elution gradient | Flow rate/sample injection volume | Detection technique | Reference |
| MS and MS/MS analyses | ||||||
| Mango seed kernel | Eluent A, water (0.01% v/v formic acid), and eluent B, acetonitrile (0.01% v/v formic acid) | C18 column 1.8 μm, 2.1 × 100 mm | 0-7 min 0-30% B; 7-9 min 30-80%; 9-11 min 80-100% B; 11-13 min 100% B and 13-14 min 0% B | 0.5 mL/min 5 μL | UHPLC-q- TOF-MS/MS ESI (-) | (Ballesteros-Vivas et al., 2019) |
| Seeds of banana passion fruit | Eluent A: water (0.01% formic acid) and eluent B: acetonitrile (0.01% formic acid) | C18 column 1.8 μm, 2.1 × 100 mm | 0 min, 0% B; 7 min, 30% B; 9 min, 80% B; 11 min, 100% B; 13 min, 100% B; 14 min,0% B | 0.5 mL/min 5 μL | UHPLC-q-TOF-MS/MS ESI (-) | (Ballesteros-Vivas et al., 2019) |
| Residues of achachairu, araçá-boi, bacaba | Eluent A (deionized water with 0.1% formic acid) and eluent B (acetonitrile with 0.1% formic acid) | Column F5 2.7 μm 2.1 x 150 mm | 0-1 min, 15% B; 1-7 min, 25% B; 7-9 min, 25% B; 9-13 min, 30% B; 13-16, 30% B; 16-21 min, 40% B; 21-23, 40% B; 23-25, 45% B; 25-28, 50% B; 28-33, 60% B; 33-37, 75% B; 37-38, 15% B | 0.2 mL/min 2 μL | UHPLC-QqQ-MS/MS | (Barros et al., 2017) |
| Lucuma seeds | Eluent A: 1% formic aqueous solution and eluent B: acetonitrile | C18column (Acclaim) 2.5 μm, 4.6 x 150 mm | time (min), % B was: (0.00, 5); (5.00, 5); (10.00, 30); (15.00, 30); (20.00, 70); (25.00, 70); (35.00, 5) and 12 min | 1.0 mL/min 2.0 - 10 μL | UHPLC/ESI/MS/M | (Guerrero-Castillo et al., 2019) |
| Avocado peels and seeds var. Fortuna | Methanol | triangular chromatographic paper | 2 µL sample volume and 40 µL of methanol | (da Silva et al., 2022) | ||
| Peel and pulp mango (Mangifera indica L.) | Binary Eluent A: ultrapure water with 1.0% acetic acid (v/v) and eluent B: methanol | Licrhorspher@” RP 18 5 μm, 4.6 × 250 mm | 0-10 min, 100% A; 10-20 min, 70% A; 20-30 min,10% A; 30-37 min, 70% A and 37- 40 min, 100% A. | 1.0 mL/ min 20 μL | (da Silva Sauthier et al., 2019) | |
| Avocado, pineapple, banana, papaya, passion fruit (pulp, seed and peels) | Eluent A (formic acid 0.1%, in Milli-Q water) and eluent B (methanol) | Column C18 1.7 μm, 2.1 × 50 mm | 95% A and 5% B to 100% B in 8.00min | 0.20 mL/ min | UPLC-ESI (-)-MS | (Morais et al., 2015) |
| Orange peels of different citrus varieties: bahia, lima, lima-of-persian, morcote, ponkan, pera, seleta, cravo, kinkan and pomelo | Eluent A:5% (v/v) of formic acid, and eluent B: methanol | Reverse phase column (Lichrochart 100 RP-18) 5μm, 0.4 x 12.5 cm | 0 min 30%B, 20min 80%B 22-25min 30%B | 1 mL/min | (Pereira et al., 2017) | |
| Grape pomace | Eluent A: 2% (v/v) acetic acid in water and eluent B 0.5% acetic acid in water and acetonitrile (50:50, v/v) | Column C18 5 μm, 4.6 × 150 mm | 10 to 24% B (20 min), from 24 to 30% B (20 min), from 30 to 55% B (20 min), from 55 to 100% B (15 min), 100% B isocratic (8 min), from 100 to 10% B (2 min) | 0.7 mL/min 10 μL | HPLC-DAD | (Ribeiro et al., 2015) |
| Citrus by-products | Eluent A: H2O (0.1% of formic acid) and eluent B: Methanol (0.1% of formic acid) | Column C18 3 μm, 4.6 x 150 mm | 90% A (0-5 min), 20% A (5-80 min), 90% A (80-85 min), and 90% A | 0.6 mL/min | HPLC-DAD | (Barbosa et al., 2018) |
| Peel and kernel of mango | Eluent A: Formic acid-water (0.1% v/v) and eluent B: acetonitrile | Column C18 4.6 x 150 mm, 5 μm | 15% B (0 min), 25% B (0 -5.5 min), 35% B (5.5-11 min), 60% B (11-31 min), 15% B (31-31.01 min), 15% B (31.01-35 min) | 0.5 mL/min 10 μL | LC-MS | (León-Roque et al., 2023) |
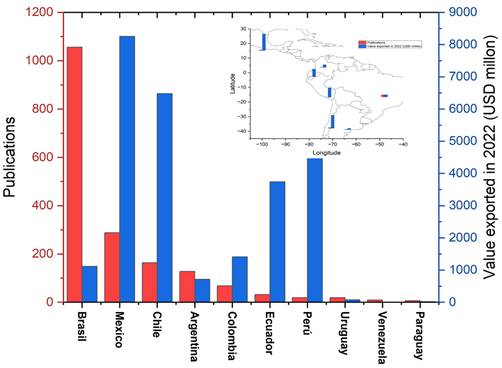
Figure 5 Fruit export volumes from Latin American countries vs. scientific production on the determination of flavonoids in fruit waste.
Most of the studies used by-products of traditional fruits such as mango, grapes, and apples, among others, and few of these studies used native or non-traditional fruits. This is basically due to the lack of commercial interest and the fact that many raw materials are seasonal (once or twice a year), but, currently, more exotic fruits are being valued for their potential and it is expected that this will attract the attention of economic groups that promote research. It is important to start working on methods for this waste, since revaluing them based on their flavonoid-rich composition means having clear techniques and procedures, such as HPLC-MS, for their adequate analysis and the subsequent development of products that may generate greater income for Latin American countries.
5. Conclusions and future studies
Studies on flavonoid compounds have aroused interest due to their pharmacological potential for human health and the possibility of being isolated from plant by-products such as fruits that are used by agro-industrial companies. In this sense, this review compiled studies carried out in Latin American countries using fruit waste and identifying flavonoids by HPLC-MS techniques, taking into account the pretreatments, the extraction technique, and important parameters in the quantification of this compound. Studies on fruit waste in Latin America are increasing, but not at the same rhythm in all fruit exporting countries, so many flavonoids with technological and bioactive potential are not being adequately identified. It is also very important to determine the optimal methods of extraction and identification of these metabolites, looking for the best yield and the generation of methods that can obtain high-quality and economically viable compounds.