1. Introduction
Since ancient times, lichens have been used as natural dyes since pre-Hispanic cultures (Dillman & Cooper, 2010) and up to the present day (Richard son, 2019; Shukla & Upreti, 2015) to dye fibers used in looms and local ornaments. In nature, they can be found in various colors, from purple, red, brown, green, and yellow, which are generated thanks to the lichen symbiosis process and have a particular affinity for natural fibers (Yamamoto et al., 2015).
Geolashon (T. flavicans) is a yellow lichen (Figure 1a) that grows naturally in the highlands of the Andes at more than 4,000 meters above sea level, where the cold is intense (reaching 8 °C in winter), and there is constant rainfall. The plants usually develop under the rocks and beside the hichu (straw), as shown in Figure 1b. Traditionally, local people use it as a source of natural pigments for their looms and in infusions for medicinal purposes.
In the last two decades, the search for natural color sources has been a latent concern in different areas of knowledge, only in 2022 there were more than 453 publications related to the topic "natural pigment", which were added to the almost five thousand existing ones (Figure 2a). Regarding lichens as a source of pigments, to date, about 246 studies have been carried out, mainly focused on medicine, biochemistry, and genetics (Figure 2b).
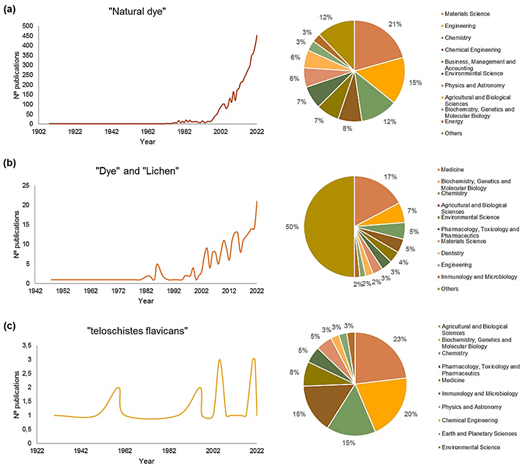
Figure 2. The trend in publications* (until December 2022) on (a) Natural pigments, (b) Lichen pigments, and (c) Teloschistes flavicans (Geolashon). *Searches conducted on February 17, 2023, in the Scopus database, using the search equations (a) “natural dye”, (b) “Dye” AND “lichen”, and (c) “Teloschistes flavicans”.
It has been discovered that the dyes produced by the symbiosis of lichens have specific chemical components with bioactive and antimicrobial capacity that are difficult to find in other natural sources; however, these metabolites may vary depending on the type and area of growth of the plant (Yamamoto et al., 2015; Yusuf, 2020). Although in the case of the Peruvian Geolashon scientific studies are limited (Figure 2c), it is known that T. flavicans contain secondary metabolites such as vicanicin that has antioxidant (Shameera Ahamed et al., 2019), antimicrobial, anticancer (El-Garawani et al., 2020) and antidiabetic (Maulidiyah et al., 2020b), which the industry can use.
In this context, this work aimed to compile the studies on Geolashon (T. flavicans) as a natural source of yellow pigments, showing its main characteristics, pigment extraction methods, bioactive properties, and as traditional applications and perspectives. In this way, the present study aims to offer scientific and traditional knowledge of this plant, showing the opportunities for its application.
2. General characteristics of Geolashon (T. flavicans)
2.1. Botanical aspects
Geolashon is a lichenized fungus belonging to the Teloschistaceae family, genus Telosquistes and species T. flavicans (NBN Atlas, 2021). It is also often called the "golden-haired lichen" in books and documents due to its morphological characteristics.
In Peru, Geolashon grows naturally mainly in the mountains of the central Andean regions of the country, such as Junín, Cusco, Huancavelica, and Ayacucho; however, it has also been reported in Cajamarca and La Libertad (Rodríguez et al., 2017). The lichen is composed of leaves or filaments of a medium-intense yellow color that, when growing on rocks, form a dense and fine layer between 1 to 2 cm thick (Figure 1b).
2.2. Collection method
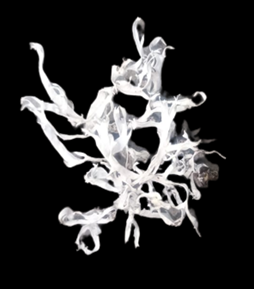
The collection is carried out in winter (September to April) when the plant is intensely yellow. Manually, the inhabitants easily extract the Geolashon from the rocks and place them in bags stored and protected from sunlight. It is unnecessary to carry out a previous drying since the lichens dry naturally in the environment. Likewise, they are kept whole, preserving the roots so as not to cause damage to the lichen. This procedure is carried out in the early hours of the day, before sunrise, to avoid color loss. Sometimes it may happen that the plant does not develop the expected pigments during its growth, so it remains white (Figure 3); this usually happens in low rainy seasons, which is estimated to be due to the lack of water during its growth. These colorless lichens are discarded since they do not help obtain pigments for craft purposes, which shows us how vital rain is for the inhabitants.
2.3. Pigment extraction and traditional applications
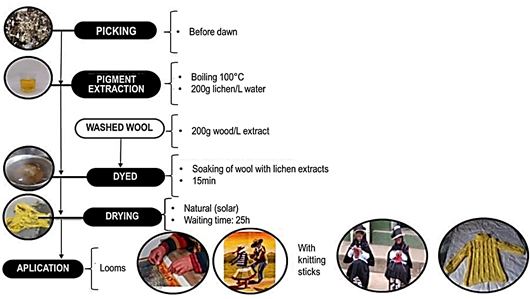
Traditionally, Geolashon pigments are extracted by boiling (100°C) the lichen in water. Previously, the lichen is washed to remove any imperfections or impurities (leaves with spots, soil, and roots) that it may contain. Then, it is submerged in hot water for approximately 15 minutes to extract the pigments. Finally, the extract is allowed to cool, which allows a better appreciation of the yellow-gold color of the extracted pigments, which are ready for use (Figure 4). In San Pedro de Cajas (Junín) district, the Geolashon extract is applied mainly in crafts and natural medicine.
2.3.1. Crafts
In its application in crafts, it is used as a natural pigment to dye wool fibers, with which it has a great affinity; following a series of steps is necessary, as shown in Figure 5. For each liter of extract, 200 g of dry lichen is used, which is used to dye 200 g of wool, although these proportions can be modified depending on the intensity of the color desired. Then, while the extract is still boiling, the sheep's wool (previously washed with boiling water and detergent) is incorporated for 15 minutes until it acquires a yellow color. The dyed wool fiber is then dried in the sun for a day to be spun and used both in the manufacture of comb looms (rugs) or for the weaving of garments.
2.3.2. Natural medicine
In the Peruvian Andes, Geolashon is used by the inhabitants as a natural antibiotic to counteract infection, stomach upset, and colic. This custom dates from the time of the Incas, who applied crushed lichen on wounds for healing, and they also made concentrated infusions of the plant.
Today, some studies support some of the properties of Geolashon. Maulidiyah et al. (2021b) and Maulidiyah et al. (2021a) determined that the T. flavicans extract has a high concentration of 3-[1'-(2",3"-dihydroxy phenyl)- propyl]-7hydroxy-chroman-4-one which has a high antioxidant and antifungal capacity. While Pereira et al. (2010) verified that the extract is not toxic and, on the contrary, has anti-edematogenic activity. Finally, Maulidiyah et al. (2020b) demonstrated the presence of high doses of vicanicin in the extracts, which has an anti-inflammatory and antidiabetic effect. Although the studies to date show promise, it would be necessary to carry out a verification through in vivo techniques that demonstrate this potential, thus opening up an opportunity for the incorporation of the lichen into other fields, such as medicine or the food industry, as a natural pigment with health benefits.
3. Geolashon as a source of yellow colorant/pigment
Color is an essential sensory attribute in all the products we consume. Depending on the product type, color may become more important for its acceptance by consumers. Currently, the sources of dyes can come from natural (Yadav et al., 2023) and artificial (Forgacs et al., 2004; Kucharska & Grabka, 2010) sources for use in food (Echegaray et al., 2023; Zhang et al., 2023), cosmetics (Vázquez-Ortega et al., 2020), drugs (Tiwari et al., 2022), textiles (Ayele et al., 2020), and other products (Jelonek et al., 2020; Prokein et al., 2021).
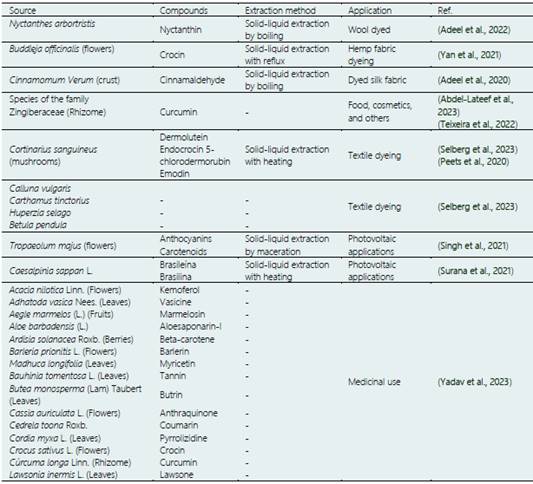
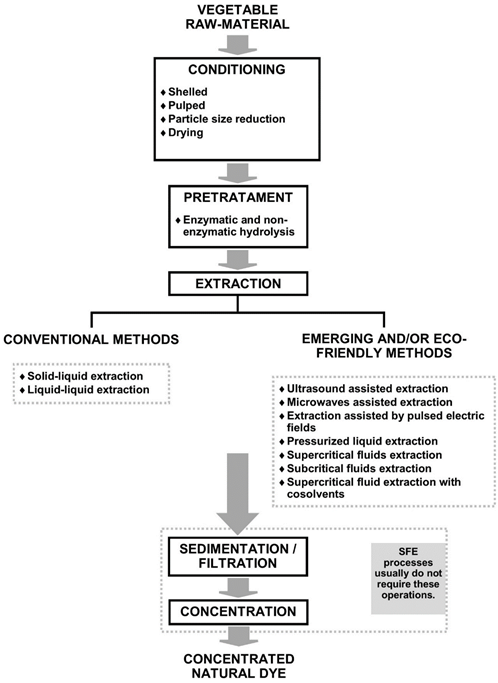
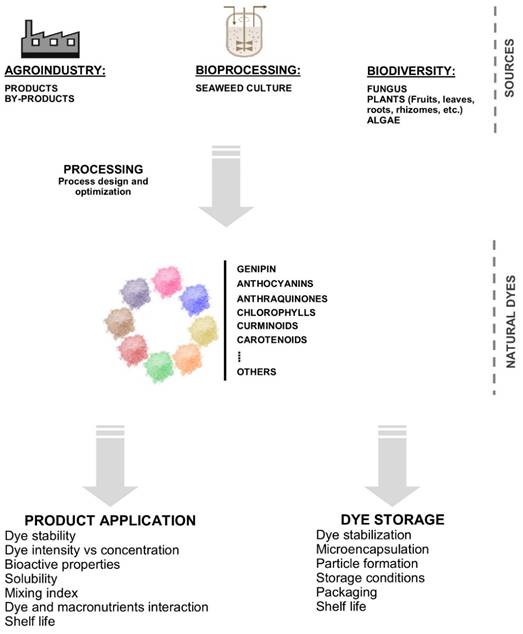
This section reviews the sources of yellow dye used in different industries. The yellow colorant is obtained from natural sources (Gandía-Herrero & García-Carmona, 2013) and can be synthesized (Rovina et al., 2016). Table 1 shows some recently reported sources of natural colorants, such as plants and fungi. Plants are a promising source of yellow pigment, rhizomes (Abdel-Lateef et al., 2023), flowers (Yan et al., 2021), leaves (Yadav et al., 2023), crust (Adeel et al., 2020), fruits (Yadav et al., 2023), and berries (Yadav et al., 2023) are used. Section 4 describes the extraction methods used for pigment/dye extraction, highlighting conventional and emerging techniques. Curiously, for extracting the natural yellow dye from different plants (Table 1), recently reported, they use the solid-liquid method, combined with high temperatures and a solvent reflux system because generally low temperatures are preferred to obtain phytochemicals. Techniques for recovery of bioactive (as natural col orants), such as pulsed electric fields - PEF (Pataro et al., 2020), ultrasound-assisted extraction - UAE (Bhimjiyani et al., 2021), microwave-assisted extraction - MAE (Sharma & Dash, 2022), and sub / supercritical fluids (Chañi-Paucar et al., 2022a), are especially interesting for the recovery of phytochemicals, mainly because they increase the extraction yield, speed up the extraction process, use GRAS solvents - Generally Recognized As Safe, and are recognized as eco-friendly techniques. The latter represents a promising perspective for producing natural dyes using more efficient and safer processes for the environment.
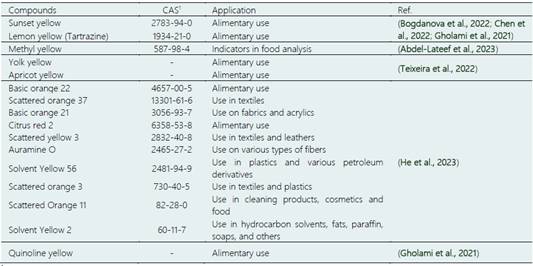
On the other hand, technologists and scientists have developed different compounds or additives for products from various industries. Dyes are a small group of those synthetic compounds that industries demand to produce their products. In Table 2, some types of synthetic yellow dyes used in different industries and their applications, recently reported, are listed. Synthetic yellow dyes provide high-quality coloring to products; they are also easy to apply and have high stability before and after application. Despite synthetic dyes' advantages, the side effects of consuming products made with these dyes have been discovered in recent years. Various studies have been developed for their extraction (Bogdanova et al., 2022; Chen et al., 2022; Gholami et al., 2021) and quantification (Gholami et al., 2021; He et al., 2023) to perform appropriate quality control. Although synthetic dyes' adverse effects are known, they cannot be stopped from being used, probably due to economic implications. Still, the scientific community has been trying to replace them with natural dyes (Teixeira et al., 2022) to contribute to a healthier life for consumers.
The knowledge of new sources of natural dyes for industrial use is a current concern. As shown in Table 1, there is a great diversity of natural sources, but not all of them have the potential for commercial use, or the conditions for their use do not exist in commercial production. Therefore, scientific research focuses on expanding the diversity of sources of natural colorants, production agronomic, and application. One way to expand the knowledge of new natural dye sources is to use an empirical understanding of the populations; in this context, a potential source of yellow dye is the lichenT. flavicans. It has been used ancestrally in San Pedro de Cajas, Tarma province, Junín department of Peru; in this city, it is used to dye artisanal textiles (de Mayolo, 1989). In San Pedro de Cajas, known as Rashta or Geolashon, this species grows from 850 to 3190 meters above sea level, and its properties have been studied in different countries (Table 3). Table 3 shows the most recent studies on the species, its applications, composition, and production zone. As is evident, the raw materials' dyeing properties and active principles may vary depending on the production area (
Table 3), therefore, their applications.
4. Extraction methods of natural pigments
Natural coloring pigments are widely used in preparing functional foods and beverages. Therefore, extraction methods are required to allow an excellent bioactive recovery with good stability (Nirmal et al., 2021).
4.1. Conventional methods
Conventional pigment extraction techniques employ toxic solvents and operations such as pressurized extraction, continuous diffusion, fermentation, maceration, boiling, grinding, and pressing (Nirmal et al., 2021).
Table 3. Uses and composition of T. flavicans
| Country | Uses | Compounds | Ref. |
| Indonesia | Antimicrobial activity against E. coli, S. typhi, K. pneumoniae, S. aureus, B. cereus, and C. albicans. | 3-[1’-(2”,3”-dihydroxy-phenyl)-propyl]-7-hydroxy- chroman-4-one | (Maulidiyah et al., 2021a) |
| Indonesia | Termiticidal activity against Coptotermes curvignthus Holmgren. | Vicanicin Methyl oleate Methyl palmitate Patchouli alcohol | (Avidlyandi et al., 2021) |
| Brazil | Potential applications for the production of food products | Polysaccharides | (Ruthes et al., 2008) |
| Indonesia | Antioxidant activity | 3-[1'-(2",3"-dihydroxy-phenyl)-propyl]-7-hydroxy- chroman-4-one | (Maulidiyah et al., 2021b) |
| Argentina | Photosensitizing properties in photodynamic reactions for cancer treatment | Parietin | (Cogno et al., 2020) |
| Indonesia | Antimicrobial activity against Staphylococcus aureus and Pseudomonas aeruginosa | Not reported | (Darwis et al., 2021) |
| Indonesia | Antileukemic activity | 2,7-dichloro-3,8-dimethoxy-1,6,9-trimethyl-11H-dibenzo[b,e][1,4]dioxepin-11- one Rhizonic acid Parietin Vicanicin | (Sanjaya et al., 2020) |
| Argentina | Antibacterial activity by photosensitization | Parietin | (Comini et al., 2017) |
| Brazil | Potential applications for the production of food products | Polysaccharides | (Reis et al., 2002) |
| Indonesia | Antidiabetic activity | Vicanicin | (Maulidiyah et al., 2020b) |
Conventional process techniques are still applied to extract pigments using water and other solvents. The extraction process can be optimized based on a specific phytochemical component to obtain better bioactive properties (Guiné et al., 2019). The toxicity of the solvents used in conventional methods limits the use of bioactive extracts. On the other hand, GRAS solvents can be used in conventional methods; with proper optimization of the process, a suitable extraction yield can be obtained, but stability and its application may be limited without prior stabilization of the bioactive (Chañi-Paucar et al., 2020). Emerging technologies are available for the production of bioactive compounds in a more efficient and eco-friendly way (Lao et al., 2020).
4.2. Emerging methods
In recent years, new pigment extraction techniques and methods have been developed, the most emerging being extraction assisted by microwave radiation, ultrasound, pressurized liquid, pulsed electric field, supercritical fluid, extraction methods that use green chemistry, techniques which proved to have excellent yield and conservation of the extracts (Miranda et al., 2021). These processes are highly efficient in recovering bioactive components from various raw materials, requiring less solvent volume, energy, and extraction time (Nirmal et al., 2021). Their principle is based on high mass transfer and increased tissue cell permeability (Nirmal et al., 2021).
4.2.1. Pulsed electric field
Pulsed electric field (PEF) technology has emerged as a promising non-thermal technique for the recovery and production of natural dyes from food matrices; its principle of action has an electro poration effect on cell structures, which makes this method an intelligent alternative for the production of natural pigments (Bocker & Silva, 2022).
Extraction assisted by the pulsed electric field (PEF), a non-thermal technique, manages to recover heat-labile bioactive compounds from plant resources (Kamboj et al., 2022). The optimization of PEF parameters (Electric Field Intensity (EFS) and Pulse Width (PW)) demonstrated a significant increase in the yield of protein content, antioxidant activity, and total phenol content, valuable compounds with potential pharmaceutical and nutraceutical applications (Kamboj et al., 2022).
4.2.2. Ultrasound
Ultrasound-assisted extraction (UAE) allows the recovery of bioactive compounds from agri-food resources. Optimizing energy consumption, time, and its principle of chemistry and green technology generates the most negligible impact on the environment (Fraterrigo Garofalo et al., 2022). This technique proved to be an alternative, novel, and efficient extraction method for industrial development, improving yield at reduced costs (Gao et al., 2022; Sengar et al., 2022). Ultrasonic technology allows the optimization of extraction and recovery parameters of high-value chemical components (Zhang et al., 2022a). Although, in some cases, ultrasound may not influence the extraction yields, as observed in the extraction of fatty acids from cumbaru seeds (Dos Santos et al., 2016). Using eutectic solvents in the ultrasonic extraction allows for considerably improved obtaining of flavonoids, total phenols, lipid peroxidation inhibition, and antioxidant capacity (Rashid et al., 2022). In the same way, the UAE method using conventional solvents allows the optimization of parameters such as extraction temperature, liquid-solid ratio, sonication time, and extraction frequencies, favoring an efficient extraction of total anthocyanins and phenolic compounds (He et al., 2016; Liao et al., 2022). During the ultrasonic extraction process, temperature and ultrasonic power density parame ters influenced the extraction yield of phenolic com pounds and chlorogenic acid from artichoke by-products (Reche et al., 2022).
4.2.3. Microwaves
Within methodologies for extraction, there is microwave-assisted extraction (MAE), which allows optimization of parameters to obtain high levels of phytochemicals with high antioxidant activity, characterizing this method as an advanced technique of high efficiency, ecological, good performance, and low energy consumption (Nguyen et al., 2020; Wei et al., 2023). The microwave-assisted extraction technique allows, through high-efficiency microwave heating, to extract bioactive compounds, making it attractive for industrial applications (Nisoa et al., 2022). The use of microwave technology proved to have better extraction performance of chemical compounds of high food value since its volumetric and selective heating effects allow it to be more efficient than other conventional heating methods (Dewi et al., 2022; Song et al., 2022). The MAE method associated with extraction parameters such as power levels, solvent concentration, and time significantly increases the yield of bioactive compounds in food and agri-food residues (anthocyanins, polyphenols, proteins, unsaturated fats, antioxidant capacity, among others) proven to be a fast, lower-cost technique that does not generate an impact on food security (Alvi et al., 2022; Aparamarta et al., 2022; Barrios et al., 2022; Boyapati et al., 2022; Pham et al., 2022).
5. Bioactive properties of pigments/extracts
Population growth has increased the demand for functional foods and nutraceutical products made with bioactive compounds (polyphenols, anthocyanins, and carotenoids) and antioxidant capacity appropriate to prevent and treat degenerative diseases (Kamatchi et al., 2022).
Recent studies report novel nitrogenous and water-soluble molecules obtained and characterized from pigments present in quinoa, and the analysis showed a notable presence of bioactive compounds with antioxidant potential with beneficial effects on health, nutraceutical potential, and an excellent alternative for applications in the food and pharmaceutical industry (Henarejos-Escudero et al., 2022) (Table 4).
Natural pigments and their usefulness as food and pharmaceutical additives are the subjects of research to identify and obtain bioactive components that protect and prevent the development of cancer and neurological damage from their functional activity of food and/or medicine (Tunca Koyun et al., 2022).
Table 4. Applications of emerging methods of pigment extraction and their functional properties
| Method | Raw-material | Compounds | Other components / Bioactive properties | Ref. |
| Pulsed Electric Field | Perilla seed flour. | Total phenolic | Proteins Antioxidant activity. | (Kamboj et al., 2022) |
| Pulsed Electric Field | tomato by-products | Carotenoids Lycopene | (Pataro et al., 2020) | |
| Pulsed Electric Field | Purple potato (Solanum tuberosum) | Anthocyanins | (Puértolas et al., 2013) | |
| Conventional aqueous extraction. Extraction assisted by infrared radiation (IR). Ultrasound (US). Pulsed electric fields (PEF). High Voltage Electrical Discharge (HVED) | Pomegranate (Punica granatum L.) peel | Polyphenols | (Rajha et al., 2019) | |
| Extraction assisted by high voltage electrical discharges | Grape cluster | Flavan-3-ols Flavonols Stilbenes | (Brianceau et al., 2016) | |
| Ultrasound assisted extraction | Pomegranate (P. granatum L.) peel | Total phenolics | Antioxidant activity. | (Sharayei et al., 2019) |
| Ultrasound assisted extraction | Citrus limetta peels | Pectin Galacturonic acid Total phenolics | Antioxidant activity | (Panwar et al., 2023) |
| Ultrasonic and microwave-assisted extractions | Sea buckthorn pomace | Lycopene β-carotene | Antioxidant activity | (Sharma et al., 2022) |
| Ultrasound assisted extraction | Linseed oil (Linum usitatissimum) | Carotenoids | Antioxidant activity | (Bhimjiyani et al., 2021) |
| Ultrasound assisted extraction | Pigmented rice bran | Anthocyanins Total phenolics Total flavonoid | Antioxidant activity | (Bunmusik et al., 2022) |
| Microwave Ultrasonic Assisted Extraction | Red corn | Technofunctional properties Organic matter absorption capacity | - | (Garcia-Ortiz et al., 2022) |
| Microwave assisted extraction | Chinese herbs | Polyphenolic compounds | Resveratrol Myricetin Safflomin A | (Wang et al., 2008) |
| Microwave assisted extraction | Paprika (Capsicum annuum L.) | Functional chemical compounds | (Csiktusnádi Kiss et al., 2000) | |
| Microwave assisted extraction | Porphyridium purpureum | B-phycoerythrin | Protein | (Huschek et al., 2022) |
| Microwave assisted extraction | Black ham pulp. | Total phenolics Total anthocyanins | Antioxidant activity Thermodynamic parameters | (Sharma & Dash, 2022) |
| Pressurized liquid extraction | Pomegranate by-products | Total phenolics α, β punicalagin Ellagic acid | (Toledo-Merma et al., 2022) |
The red and purple coloration in dark-colored potatoes expresses the high content of anthocyanins. In vitro analysis of these extracts from dark potatoes showed significant antioxidant, antibacterial, and antifungal activity values, which, when added to the formulation of beverages, present acceptable sensory profiles and better shelf life performance than other commercial dyes (Sampaio et al., 2021). The flavonoids in vine extracts proved to have excellent properties in the textile industry; good dye fixation was observed when using a natural mordant to carry out the dyeing (Zhang et al., 2022b). Natural dyes and their addition to the manufacture of food follow a line of study about their benefit in human health, replacing synthetic dyes; for this benefit, innovative extraction and stabilization techniques have been developed for their application in the food industry (Durazzo et al., 2022).
6. Natural colorants / pigments applications
6.1. Foods
Currently, the use of dyes in food has been extended to other needs in addition to providing color only, such as formulating food with active ingredients that contribute to the good health of consumers (Imchen & Singh, 2022), in the manufacture of intelligent food packaging (Latos-Brozio & Masek, 2020), and the manufacture of edible films with attractive physicochemical and sensory properties for the consumer (Sun et al., 2023). The chemical nature of natural food dyes gives them bioactive properties, such as antioxidant capacity, antidiabetic activity, anticancer activity, and others (Imchen & Singh, 2022). Algae is a promising natural source of natural dyes, which can produce various pigments, such as carotenoids, chlorophylls, and phycobiliproteins (Imchen & Singh, 2022). On the other hand, natural food dyes can be used to manufacture edible films for food (Latos-Brozio & Masek, 2020); this can be achieved by combining the dyes lycopene, phycocyanin, and curcumin with nanocrystals of cellulose and whey protein, under operating conditions and composition, it is possible to obtain adequate sensory properties and be accepted by the consumer (Latos-Brozio & Masek, 2020).
In the same way, it is possible to use natural pigments (chlorophyll, lutein, curcumin, and β-carotene) as shelf-life indicators of biodegradable innovative packaging materials (Latos-Brozio & Masek, 2020). The color changes of intelligent packaging formulated with natural pigments are capable of evidencing the state of the useful life of the food product and biodegradable packaging (Latos-Brozio & Masek, 2020). The new applications of natural colorings represent a horizon of opportunities for the healthiest and most eco-sustainable food industry.Another aspect of applying dyes in food is using healthier colorants and pigments (He et al., 2021; Latos-Brozio & Masek, 2020) and producing sustainably with environmental responsibility through the use of waste agro-industrial (Chañi-Paucar et al., 2020; Toledo-Merma et al., 2022). Recently, to incorporate healthier dyes in commonly consumed food products, pre-neutralized crude palm oil has been included as a source of yellow/orange colorant (carotenoids) for the preparation of fish sausages (Chaijan & Panpipat, 2021), anthocyanins extracted from Ficus carica peels and Prunus spinosa fruit for confectionery products (Backes et al., 2020) and yellow/orange prickly pear pulp microparticles as a source of coloring for yogurt (Carmona et al., 2021). Recently reported natural dyes to possess well-known bioactive properties, such as antioxidant and antimicrobial activity (Backes et al., 2020).
6.2. Textiles
The dyes used in textiles can be of natural and synthetic origin (Nambela et al., 2020). The textile industry's most widely used synthetic dyes are azo and carbonyl dyes (Nambela et al., 2020). Other synthetic dyes are nitro, phthalocyanine, polymethine, stilbene, sulfur, and triphenylmethane (Allen, 2013). On the other hand, the natural dyes used in the dyeing of textiles can be obtained from plants, insects, minerals, and microorganisms (Nambela et al., 2020). The natural dyes mentioned in other sections of this document can be used for the dyeing of textiles. Natural pigments can be classified into the chemical groups of carotenoids, indigoids, quinonoid, flavonoids, tannins, and betalains (Nambela et al., 2020).
Using synthetic dyes in textiles brings potential risks of teratogenicity, carcinogenicity, and environmental problems, prompting the search for healthier alternatives from natural sources (Mussagy et al., 2022a). Using natural dyes implies that the appropriate dyeing conditions and the type of mordant must be optimized. A yellow colorant obtained from Buddleja officinalis was recently used to dye hemp cloth; the suitable dyeing conditions were pH 5, 60 ºC, and 90 min (Yan et al., 2021). Depending on the type of mordant (natural or metallic), the use or not of mordant can produce different intensities of the yellow dye of B. officinalis (Yan et al., 2021). Other sources of natural colorants such as the bark of Melia azedarach (Tian et al., 2022), Phaffia rhodozyma yeast (Mussagy et al., 2022b), Ipomoea batatas agricultural residues (Fang et al., 2022), and others. In Peru, there are various plants with the potential to produce natural dyes to dye textiles, which can provide different shades of colors depending on the type of mordant (Rojas et al., 2016). The intensification of the use of these species requires studies to optimize the transformation process and production of the raw material on a commercial scale.
6.3. Cosmetics
The cosmetics industry is changing, and a strong trend is to incorporate safer additives and inputs for consumers' health (Lourith & Kanlayavattanakul, 2023; Pinto et al., 2020). In this context, the cosmetic industry has identified significant potential in active ingredients from agro-industrial waste for producing cosmetic products, replacing synthetic/artificial components (Pinto et al., 2020). Synthetic dyes are one of the components used to manufacture cosmetic products. Its constant use and accumulation in the organism can lead to various health problems and diseases (Khatun et al., 2020). An example of these dyes is ferrous salts (Fe2+) used to manufacture different widely used cosmetic products (Khatun et al., 2020). Due to the danger of metallic pigments and to prevent the accumulation of Fe2+ ions in cells and their presence in cosmetics, a diagnostic method has been developed for their detection in cosmetic products and living cells (Khatun et al., 2020).
On the other hand, cosmetic dyes can generate singlet oxygen (1O2) due to the absorption of ultraviolet radiation and visible light by the chromophore groups of the cosmetic dye molecules (Vázquez-Ortega et al., 2020). 1O2 is a reactive species capable of oxidizing biomolecules, adversely affecting skin health (Vázquez-Ortega et al., 2020). Natural dyes are presented as an attractive alternative to synthetic dyes due to their color-providing properties and bioactive properties (antioxidant, antimicrobial, anti-inflammatory, etc.), but some aspects of these dyes that limit their use must be resolved beforehand to spread their use; among the negative aspects are the low stability of natural dyes and undesirable sensory characteristics (Lourith & Kanlayavattanakul, 2023).
In addition to developing the most appropriate processes that allow the inclusion of natural colorants in cosmetic products, there is another problem of equal importance, the safe production of natural dyes. It is well known that conventional methods, especially those that use toxic solvents, limit the use of bioactive in the industry; in this scenario, high-pressure fluid technologies are presented as a good alternative for the production of bioactive for the cosmetics industry (Chañi-Paucar et al., 2022c; Zoric et al., 2022) from different plant raw materials (Chañi-Paucar et al., 2021, 2023; Chañi-Paucar et al., 2022b; Chañi-Paucar et al., 2022a; Toledo-Merma et al., 2022).
7. Current and future challenges
The natural dyes industry is growing due to increased demand from consumers, who have increased their preferences for healthier products produced with environmental responsibility. Research has revealed a great diversity of natural sources of dyes (Tables 1 and 4), with potential applications for various industries. Applying natural dyes in the production of products in different industries brings many challenges for the productive and industrial sectors. The most relevant challenges in the productive sector refer to the sustainability of the supply of these new raw materials and sources of dyes for the industry since, in some cases, they are native, endemic, and/or seasonally produced species and by-products of agro-industry. Therefore, they require adequate planning that allows their sustainability.
On the other hand, the development of technolog ical packages for production, which involves the cultivation and/or harvesting, pretreatment, and conditioning of raw materials for the processing industry, is still under research, as is the case of algae biotechnology for the production of dyes (Imchen & Singh, 2022). The challenges for the industrial processing sector refer to technological aspects of the raw materials that are sources of colorants and dyes. The processing of new raw materials, and sources of natural pigments, demands the design and optimization of adequate processes to obtain quality products with a good, helpful life that allows their distribution, commercialization, and subsequent consumption. The useful life of products made with natural products, such as natural colorants, has been the subject of various studies to improve their stability as a pure additive and in derivatives, which may contribute to extending their useful life (Bocker & Silva, 2022; Carmona et al., 2021; Chañi-Paucar et al., 2020; Miranda et al., 2021; Nirmal et al., 2021; Sampaio et al., 2021; Sun et al., 2023). Another important aspect of using new raw materials in the industry is their relevance of use, that is, how safe is the use of natural raw materials and their derivatives in daily consumer products, such as food and cosmetics? , some adverse health effects derived from the use of natural products have been reported (Hausen, 2001; Nanda & Wasan, 2016), probably due to the presence of toxic components derived from the complex composition of natural products, in this context, refinement of extraction and purification technologies for natural bioactive on a commercial scale is pending.
The use of natural colorants by the cosmetics, food, drug, and other products industry is growing, with a strong tendency to displace synthetic dyes, especially in the food and cosmetics industry. Using natural products in the industry brings opportunities for this sector, such as the positioning and development of new specialized market niches that demand products with these characteristics at a differentiated price.
8. Conclusions
The lichen T. flavicans has been used ancestrally in the towns of the central highlands of Peru, highlighting its application in the dyeing of artisanal textiles and some applications in traditional medicine. According to our review, the medicinal potential of this lichen is due to the presence of bioactive compounds such as rhizonic acid, parietin, vicanicin, and flavicansone, which present a yellowish appearance when concentrated, which probably contribute to the dyed in artisan textiles. T. flavicans is a promising source of bioactive for food, medicinal, and cosmetic applications, which until actuality, has yet to be extensively researched for industrial use. Aspects such as extraction and purification must be studied for the correct use of this resource, mainly due to possible adverse synergistic effects between its bioactive components that limit its use, due to the complexity of its composition. In this context, green technologies are currently available that can be used for the safe production of bioactive for use in manufacturing products for human consumption.