1. Introduction
Over the years, broiler productivity has increased considerably (Korver, 2023). This has been possible due to the use of antibiotic growth promoters (AGP) in poultry farming, as a preventive measure against or for the treatment of infectious bacterial diseases (Abreu et al., 2023). However, the widespread use of these antimicrobials generates resistance, with important consequences for animal health and, potentially, human health. In a recent study, it was shown that the global trend regarding the use of antimicrobials in food-producing animals will increase by 8.0% by 2030 (Mulchandaniid et al., 2023). Curiously, for years, although AGP use in birds has tended to reduce, there are still challenges to maintaining intestinal health and, consequently, the performance of birds (Korver, 2023).
Halquinol (chlorhydroxyquinoline) is a potent non-antibiotic substance renowned for its antibacterial, antifungal, and antiprotozoal properties. It is composed of a mixture of three chlorinated oxines: 5,7-dichlor-8-hydroxyquinoline, 5-monochlor-8-hy-droxyquinoline, and 7-monochlor-8-hydroxyqui-noline (Cosgrove, 1977). Halquinol is formulated into various pharmaceutical dosage forms for both human and veterinary use. It exhibits broad-spectrum antimicrobial activity by inhibiting respiratory enzymes in the cytoplasmic membrane of target organisms, distinguishing it from quinolones (World Health Organization & Food and Agriculture Organization of the United Nations, 2021). In developing countries, halquinol is commonly used as a feed additive in poultry production (Basit et al., 2020; Habib et l., 2019). While no microbiological studies have suggested the development of halquinol resistance, concerns have been raised regarding its potential impact on the colonization barrier (World Health Organization & Food and Agriculture Organization of the United Nations, 2021).
In the search for alternatives, essential oils (EOs) have emerged as promising options for improving the productive performance of various bird species, including quail, turkeys, laying hens, and Pekin ducks (Xiao et al., 2022; Ding et al., 2020; Loyaga-Cortéz et al., 2020; Mendoza-Ordoñez et al., 2020). EOs are complex mixtures of volatile compounds extracted from plants, and their effects stem from the collective action of all components and their interactions (Abd El-Hack et al., 2022). A recent systematic review has underscored the significant role of EOs in the metabolic pathways of broilers as antimicrobial, antioxidant, and anti-inflammatory agents, resulting in enhanced nutrient digestibility, carcass percentage, intestinal integrity, and metabolite profile (Irawan et al., 2021).
However, EOs are highly sensitive to factors such as high temperatures, ultraviolet light, and oxidation, which can compromise their biological activity through volatilization or degradation of active ingredients (Asbahani et al., 2015). To overcome these challenges, nanoencapsulation has emerged as a viable approach for protecting EOs from degradation and evaporation (Hasani et al., 2018). Recent studies have demonstrated that nanoencapsulation of EOs not only provides cost-effective preservation of bioactive compounds but also enhances their beneficial effects compared to free-form EOs (Amiri et al., 2020, 2021; Nouri, 2019).
In this context, the use of essential oils as substitutes for AGPs has gained great popularity among poultry breeders, due to their multiple benefits (Qui, 2023). Various researchers have shown that EOs improve productive performance and at the same time reduce the negative results of heat stress (Señas-Cuesta et al., 2023; Yilmaz & Gul, 2023), coccidiosis (Zhang et al., 2023), Chicken necrotic enteritis (Zheng et al., 2023), among others. It even appears that supplementation with nanoemulsified vegetable oil and probiotics improves the performance, carcass quality, and meat characteristics of broiler chickens (Suliman et al., 2023). Finally, although a large amount of evidence shows the positive effects of EOs, there are few studies that compare their effectiveness with the almost forgotten classic antibiotics such as halquinol (Basit et al., 2020). Nevertheless, research examining the effects of nanoencapsulated essential oil blends (N-EOs) remains limited. Therefore, the objective of this study was to evaluate and compare the efficacy of halquinol and nanoencapsulated essential oil blends in terms of growth performance, intestinal morphology, and meat quality in broiler chickens.
2. Methodology
2.1. Animal Distribution and Treatments
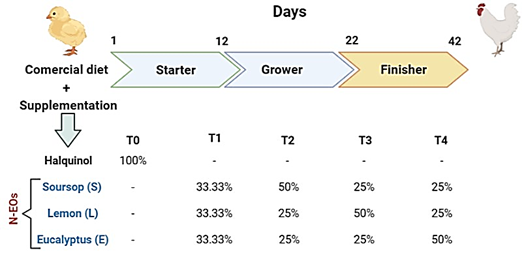
The animals were handled in accordance with the guidelines of the Code of Ethics for Research at the Universidad Nacional de Trujillo. A total of 500 male Cobb 500 broiler chickens were randomly assigned to five dietary treatments (T), with five replicates per treatment and 20 birds per replicate. All birds were housed in the same poultry pen on a local farm, with each replicate assigned to a separate pen measuring 2 x 1 m. The control group was fed a commercial diet supplemented with 60% halquinol at a dose of 70 mg/kg, while the other treatment groups (T1, T2, T3, and T4) received a diet supplemented with a dose of 75 mg/kg of a nanoencapsulated essential oil (EO) blend derived from three plants: soursop (Annona muricata), lemon (Citrus limon), and eucalyptus (Eucalyptus globulus).
Table 1 Ingredients and chemical composition of the experimental diets
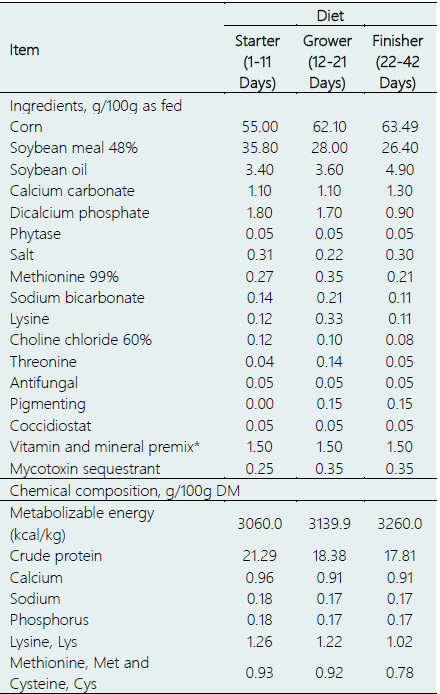
*Per kg contains 10 000 000 IU vitamin A, 3 000 00 IU vitamin D3, 15 000 IU vitamin D, 2.5 g vitamin K3, 2 g vitamin B1, 8 g vitamin B2, 4 g vitamin B6, 0.012 g vitaminB12, 20 g vitamin B5, 1.5 g vitamin B9, 70 g vitamin B3, 0.2 g vitamin B7, 70 g manganese, 70 g zinc, 80 g iron, 10 g copper, 1 g iodine, 0.3 g selenium, and excipients. DM: Dry Matter.
A detailed description of the treatments is provided in Figure 1. The chicks were raised on crushed straw litter, and each pen was equipped with nipple drinkers and plastic feeders to provide ad libitum access to feed and water. The dietary composition varied at three different growth phases: starter (1-11 days), grower (12-21 days), and finisher (22-42 days), as shown in Table 1. The nutrient composition of each basal diet was formulated to meet the chicks' nutritional requirements based on feed composition and nutritional requirement tables (Rostagno et al., 2017).
2.2. Nanoencapsulation of Essential Oils
The essential oil (EO) from soursop seeds was obtained using the extrusion method. Soursop seeds were collected, washed, and dried in an oven at 70 °C for two days. They were then subjected to pressing at 100-105°C using a screw (Lee et al., 2011). The essential oils from lemon and eucalyptus leaves were obtained through steam entrainment. Once the oils were obtained, nanoencapsulation was performed following a previously described method (Fioramonti et al., 2019) with some modifications. In summary, a primary emulsion was prepared by mixing 18% (w/w) oil phase with 82% (w/w) 4% casein aqueous phase at pH 7 using a high-speed mixer (Witeg, Germany) for 30 seconds at the highest speed. The pre-emulsion was then subjected to two passes of high shear fluid homogenization at room temperature (25°C) using a microfluidizer (Microfluidizer LM10, USA) to reduce the droplet size to nanometer levels. The primary emulsion was subsequently diluted, and the pH was adjusted with 0.3N HCl to form secondary emulsions. To create the interfacial double layer, the secondary emulsions were further diluted by adding maltodextrin solutions to increase the solids content. Finally, nanocapsules of the blend of soursop, lemon, and eucalyptus essential oils were obtained through dehydration of the secondary nanoemulsions via spray drying (Techno Search Process & Systems - India), which have 40% of essential oils.
2.3. Growth Performance
On day 1 of the experiment, all chicks were individually weighed to obtain their initial weights. Average daily growth rate and feed consumption were recorded for each replicate throughout the study. Feed conversion ratio was calculated at the end of the study.
2.4. Slaughter Procedures and Meat Sampling
On day 42 of the trial, after a 12-hour fasting period, the broilers were transferred to the slaughterhouse. The slaughter and hygienic processes were conducted in accordance with the poultry sanitary system standards outlined in Peruvian Resolution No. 093-2010-AG-SENASA. Carcasses were automatically processed (plucked, head and feet separated, eviscerated) while maintaining the cold chain. The carcasses were weighed and graded to determine carcass yield, breast yield, and abdominal fat yield. Following the slaughter process, chicken carcasses (pectoralis major muscle for texture profile analysis and thigh for oxidative stability analysis) were randomly selected from each treatment, including the control. The samples were vacuum-packed in oxygen-impermeable bags and transferred to the laboratory in refrigerated chambers. Each pectoralis major muscle sample was divided into six aliquots. Two aliquots per test were used to determine texture profile, bromatological composition, and pH. Thigh samples were stored for 15 days to evaluate oxidative stability.
2.5. Intestinal morphology
At 14, 28, and 42 days of age, two chickens per pen were sacrificed, and 1 cm samples were collected from the duodenum, jejunum, and ileum. The samples were washed with cold saline solution, weighed, and preserved in 10% formalin for 48 hours. The tissue samples were dehydrated using a series of alcohols with increasing concentrations and embedded in kerosene using a kerosene embedding center (Microm EC 350). Sections measuring 5 μm in thickness were stained with hematoxylin and eosin. The length and width of the villi were measured from the tip of the villi to the valley between individual villi, and the crypt depth was measured from the valley between individual villi to the basolateral membrane. Five villi were measured for each sample. Images were captured using an optical microscope (Zeiss Axiolab), and a computerized image analysis system (Zen Blue Edition 3.0) was utilized to obtain the data.
2.6. Texture profile
The textural properties of the samples were analyzed using a TA.HD Plus dual-column texture analyzer (Stable Micro Systems Ltd., UK). Texture tests were conducted using the Texture Exponent software version 6.1.5.0 (Stable Micro Systems Ltd.). Fifteen chicken breast samples were prepared with defined sizes and weights (height: 10 mm, length: 50 mm, width: 30 mm). The same preparation procedure was applied to all samples analyzed in the texture tests. Tests were performed in triplicate per treatment. The puncture test was performed using a 2 mm diameter cylindrical probe, a 5 kg load cell, a test speed of 1.5 mm/s, two cycles, a deformation distance of 5 mm, and a compression time between cycles of 3 seconds. Peak strength correlates with sample hardness. The specimen was placed in the center under the probe during the test. The firmness of the specimens was determined through a shear test. The shear test employed a Warner-Bratzler (WB) shear blade, a 100 kg load cell, a shear rate of 2 mm/s, and a deformation distance equivalent to 10 mm.
2.7. Bromatological Analysis and pH Determination
During the trial, a total of nine feed samples (three samples per feeding period) were taken and analyzed. The samples were initially vacuum-packed and stored at 20°C until analysis. The feed samples were analyzed for dry matter, metabolizable energy, crude protein, calcium, sodium, phosphorus, lysine, and methionine following the guidelines provided by the Association of Official Analytical Chemists (AOAC, 2019).
Chicken breast samples were evaluated for dry matter, crude protein, and ethereal extract using the same analytical methods. The bromatological parameters were determined according to the AOAC (2019).
pH determination was conducted following the method described by Honikel (1998). A meat sample was pierced, and an electrode was inserted perpendicular to the muscle mass at a depth of approximately 2 cm, avoiding contact with fat or connective tissue. The pH measurement was recorded, and the electrode was removed. Subsequent readings were taken on different parts of the same muscle after cleaning the electrode. Three readings were taken on each sample, and the electrode was periodically checked for proper functioning.
2.8. Oxidative stability
Oxidative stability was measured with slight modifications to the method described by Mendoza-Ordoñez et al. (2020). Two grams of thigh meat were weighed and homogenized in 20 mL of deionized water using a homogenizer at 13,500 rpm for 1 minute. Then, 2.5 mL of cold 25% (w/v) trichloroacetic acid (TCA) was added to the homogenate, followed by centrifugation for 15 minutes at 6000 rpm and 4 °C. A 3.5 mL aliquot of the supernatant was transferred to a new test tube, and 1.5 mL of 0.6% (w/v) aqueous 2-thiobarbituric acid (TBA) was added. The mixture was incubated at 70 °C for 30 minutes. After cooling, the absorbance was measured at 532 nm against a blank consisting of 2.5 mL of deionized water, 1 mL of 25% aqueous TCA, and 1.5 mL of 0.6% TBA. A calibration curve was constructed using n 1,1,3,3-tetraethoxypropane as a standard. The results were expressed in nmol/g tissue.
2.9. Statistical Analysis
To assess the effect of the dietary treatments, data on yield indicators, texture profile, pH, broma-tological analysis of chicken breasts, and oxidative stability were analyzed using one-way or two-way analysis of variance (ANOVA) as appropriate. Differences between means were evaluated using Tukey's multiple comparisons test. A significance level of p ≤ 0.05 was considered statistically significant. The data are presented as mean ± standard error of the mean (SEM). All statistical analyses were performed using GraphPad Prism software (San Diego, CA, USA).
3. Results and discussion
3.1. Growth Performance
The effects of supplementing the diet with nanoencapsulated essential oil (N-EO) combinations on the growth performance of broiler chickens are presented in Table 2. Throughout the 42-day experiment, broilers fed the T1 and T4 diets exhibited significantly higher average daily growth rates (p < 0.05) compared to the control group that received halquinol supplementation. Similarly, the T1 and T4 groups showed improved feed conversion ratios, with significantly lower values than the control group (p < 0.05). In terms of carcass yield, all N-EO supplemented groups demonstrated significantly higher yields compared to the control (p < 0.05), except for the T3 group, which showed similar breast yields to the control. However, there were no significant differences in daily feed intake between the N-EO supplemented groups and the control group (p > 0.05). Additionally, while the T2 group tended to reduce abdominal fat yield, there was no significant improvement in this parameter compared to the control group. In fact, the T2 group showed an increase in abdominal fat yield (p < 0.05).
The search for effective alternatives to antibiotic growth promoters (AGPs) is a global priority. Essential oils (EOs) extracted from plants and spices are being recognized as potential alternatives due to their high safety and beneficial biological effects. However, while several studies have shown positive effects of EOs on the growth performance of poultry (Ding et al., 2020, 2017; Loyaga-Cortéz et al., 2020; Mendoza-Ordoñez et al., 2020), most of these studies have evaluated the individual effects of EOs. The effects of EO combinations on poultry performance have yielded controversial results compared to control treatments (Cetin et al., 2016; Hesabi Nameghi et al., 2019; Sidiropoulou et al., 2020), and some studies have even shown no beneficial effects (Reyer et al., 2017).
Moreover, the sensitivity of EOs to high temperatures, ultraviolet light, and oxidation is a significant challenge that needs to be addressed to preserve their biological activity. Recent studies have demonstrated that nanoencapsulation can enhance the efficacy and stability of EOs, surpassing the effectiveness of AGP treatments (Amiri et al., 2020, 2021). In this study, the dietary supplementation of nanoencapsulated EOs showed positive effects on average daily growth rate, feed conversion rate, carcass yield, and breast yield. These findings are consistent with previous studies that have highlighted the antioxidant, antimicrobial, immunomodulatory, and enzyme secretion properties of EOs, leading to improved growth performance and feed efficiency in broiler chickens (Di et al., 2022; Hesabi Nameghi et al., 2019; Zeng et al., 2015).
Table 2 Effect of halquinol and nanoencapsulated essential oils (N-EOs) supplementation on performance indicators of broiler chickens
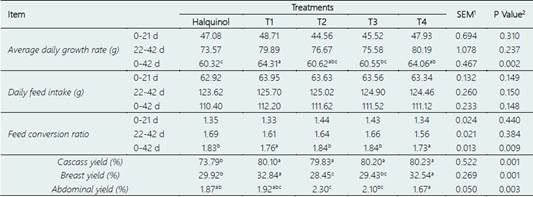
Treatments include - Control group: received a conventional diet supplemented with halquinol (60%). The experimental groups (T1-T4) received a diet supplemented with N-EOs from soursop (S), lemon (L) and eucalyptus (E) in different proportions (%): T1 (S:33.33%, L:33.33% and E:33.33%), T2 (S:50%, L:25% and E:25%), T3 (S:25%, L:50% and E:25%) and T4: (S:25%, L:25% and E:50%).
a,b,cMeans with different superscripts within columns differ significantly (P < 0.05).
1SEM: standard error of the means.
2P-values associated with dietary treatment.
3.2. Intestinal morphology
The effect of different N-EO combinations on the small intestine morphology of broiler chickens at 14, 28, and 42 days is shown in Figure 2. At the duode num level, on day 14, the T1 and T4 treatments resulted in significantly greater villus height (p < 0.01) compared to the control group that received halquinol supplementation. The T1 and T2 groups also exhibited greater villus width (p < 0.05 and p < 0.01, respectively). However, no significant differences were observed in crypt depth and villus height/crypt depth ratio between the N-EO supple mented groups and the control. On day 28, the T4 group showed a significantly greater crypt depth compared to the control (p < 0.05). At day 42, only the T1 group significantly increased villus height (p < 0.05) and villus height/crypt depth ratio (p < 0.01) compared to the control.
In the jejunum, on day 14, no significant changes (p > 0.05) were observed between the treatments. On day 28, the T1 group exhibited greater villus height (p < 0.05), while the T3 (p < 0.05) and T4 (p < 0.01) groups significantly increased villus width compared to the halquinol-treated group. Crypt depth and villus height/crypt depth ratio showed no significant differences between the N-EO supplemented groups and the control (p > 0.05). At day 42, all N-EO supplemented groups obtained greater villus width compared to the control (p < 0.05), while there were no significant differences in the other parameters between groups. Like the jejunum, no significant morphological changes (p > 0.05) were observed at the ileum level on day 14 of the trial. On day 28, the T1 and T3 groups showed increased villus height compared to the control (p < 0.001 and p < 0.01, respectively). The T2 group achieved greater crypt width (p < 0.05) compared to the control.
Regarding crypt depth and villus height/crypt depth ratio, only the T1 group showed significant improvements compared to the control group (p < 0.05). At day 42, like the jejunum, all experimental groups that received a diet supplemented with N-EOs obtained a greater villus width compared to the halquinol-supplemented group (p < 0.01).
Broiler chickens fed a diet supplemented with nanoencapsulated EOs exhibited significantly higher and wider intestinal villi in the duodenum, jejunum, and ileum compared to those fed a diet supplemented with halquinol. This improvement in intestinal morphology is attributed to the ability of EOs to regulate the intestinal microbiota, increase nutrient absorption, and promote epithelial cell mitosis, thereby increasing the surface area for nutrient absorption (Peng et al., 2016; Skoufos et al., 2016).
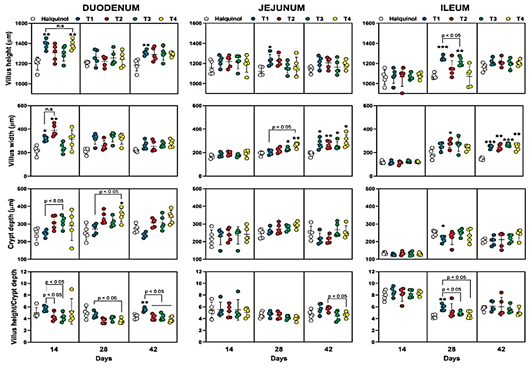
Figure 2 Effect of halquinol and nanoencapsulated essential oils (N-EOs) supplementation on the intestinal morphology of broiler chickens on the age of days 24, 28 and 42. Control group: received a conventional diet supplemented with halquinol (60%). The experimental groups (T1-T4) received a diet supplemented with N-EOs from soursop (S), lemon (L) and eucalyptus (E) in different proportions (%): T1 (S:33.33%, L:33.33% and E:33.33%), T2 (S:50%, L:25% and E:25%), T3 (S:25%, L:50% and E:25%) and T4: (S:25%, L:25% and E:50%). Each circle represents an individual value, in addition the horizontal line is the average value, and the vertical line represents the standard error of the mean (n = 5). n.s = not significant, statistical differences: * P < 0,05; ** P < 0,01; *** P < 0,001 vs. control (halquinol).
The controlled release of active compounds from nanoencapsulation enables the preservation of their properties at low concentrations, leading to superior effects on intestinal microarchitecture (Amiri et al., 2021). Likewise, the results obtained are consistent with the results of the study by Wang et al. (2007) and Prakatur et al. (2019), who demonstrated that chickens fed a diet supplemented with a mixture of bee pollen had significantly higher and wider intestinal villi of the duodenum, jejunum, and ileum in comparison to the chickens fed a control diet. The same authors further determined that the observed differences were greater during the early stages of development of the gastrointestinal system (Prakatur et al., 2019; Wang et al., 2007). Tekeli et al. (2010) showed that the addition of ginger and propolis extract both separately and in combination in the diet resulted in a significant increase in the length of the intestinal villi of the jejunum in chickens from the experimental groups when compared to chickens of the control group. Eyng et al. (2014) showed that the intestinal villi of the duodenum of chickens fed a diet supplemented with propolis were shorter or lower when compared to the intestinal villi of the chickens in the control group. Within the explanation of the identified influence of nanoencapsulated essential oils on the histological features of chickens’ intestines, it is important to keep in mind that diet composition is in fact the main factor that can modify the histological appearance or morphology of the intestine and, consequently, its absorptive capacity, which ultimately defines the growth performance of fattening chickens (Prakatur et al., 2019). It is further known that the intestinal villi are quickly and continuously adjusted as a response to conditions in the lumen of the intestine (that are strongly influenced by diet composition) reflecting the dynamic environment inside the intestines of animals. Accordingly, longer intestinal villi are associated with an increase in the absorptive surface of the intestines and with an increase of the absorption capacity of the intestine (Prakatur et al., 2019). This finding was also demonstrated in the present study, since the absorptive surface area of duodenal villi in all experimental groups were increased in comparison to that of the control broilers.
3.3. Texture profile
The texture profile results are reported in Table 3. Broiler chickens supplemented with N-EOs exhib ited higher breast meat hardness compared to those supplemented with halquinol (control group). The T1 and T4 groups obtained the highest hardness values (p < 0.05). However, there were no significant differences in toughness, firmness, adhesiveness, elasticity, cohesion, gumminess, chewiness, and resilience between the N-EO supplemented groups and the control (p > 0.05). Notably, the T2 group obtained the lowest hardness value (p < 0.05) among all treatments.
Texture profile analysis is an essential aspect of meat quality evaluation, and the texture characteristics of meat influence consumer perception. In our study, broiler chickens supplemented with nanoencapsulated EOs exhibited higher hardness values in breast meat compared to the control group. This suggests that the EOs positively affected meat hardness. These findings align with a previous study that reported improved meat quality, specifically increased meat toughness, in broilers supplemented with grape pomace in diets enriched with polyunsaturated fatty acids (Turcu et al., 2020). However, there is limited research on the effects of EOs on texture profile analysis in broiler meat, and results have been inconsistent, likely influenced by factors such as administration form, broiler line, and growth period of EO supplementation (Cázares-Gallegos et al., 2019; Hernández-Coronado et al., 2019; Alfaig et al., 2013).
Table 3 Effect of halquinol and nanoencapsulated essential oil (N-EOs) supplementation on texture profile of broiler chickens breast meat
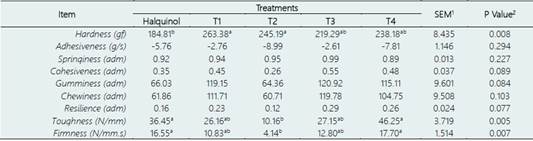
Treatments include - Control group: received a conventional diet supplemented with halquinol (60%). The experimental groups (T1-T4) received a diet supplemented with N-EOs from soursop (S), lemon (L) and eucalyptus (E) in different proportions (%): T1 (S:33.33%, L:33.33% and E:33.33%), T2 (S:50%, L:25% and E:25%), T3 (S:25%, L:50% and E:25%) and T4: (S:25%, L:25% and E:50%).
a,b,cMeans with different superscripts within columns differ significantly (P < 0.05).
1SEM, standard error of the means.
2P-values associated with dietary treatment.
3.4. Bromatological analysis and pH determination
The texture profile results are reported in Table 4. Broiler chickens supplemented with N-EOs exhibited higher breast meat hardness compared to those supplemented with halquinol (control group). The T1 and T4 groups obtained the highest hardness values (p < 0.05). However, there were no significant differences in toughness, firmness, adhesiveness, elasticity, cohesion, gumminess, chewiness, and resilience between the N-EO supplemented groups and the control (p > 0.05). Notably, the T2 group obtained the lowest hardness value (p < 0.05) among all treatments.
Bromatological analysis of chicken breast meat revealed that the nanoencapsulated EO supplementation resulted in higher crude protein percentages compared to the control. This is consistent with a study that used a mixture of thyme, oregano, and rosemary EOs at different concentrations (Popović et al., 2019). However, other studies have reported no significant differences in bromatological parameters between EO-supplemented groups and the control (Giannenas et al., 2016). The T4 group, which had the highest proportion of EO supple mentation, showed lower fat percentages in breast meat. Some compounds present in EOs have been reported to reduce fat accumulation by inhibiting cholesterol synthesis. For instance, EOs have been found to induce the activity of pyrophosphatase, leading to the degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a key enzyme in cholesterol synthesis (Case et al., 1995; Bahr et al., 2021).
3.5. Oxidative stability
Oxidative stability was measured by evaluating malondialdehyde (MDA) levels as a reliable marker of lipid peroxidation (Figure 3). Broiler chickens fed a diet supplemented with halquinol exhibited the highest levels of MDA (10.14 ± 2.6 nmol/g tissue). In contrast, broilers that received a diet supplemented with N-EOs had lower levels of this oxidative stress marker. The T1 group obtained the lowest levels of MDA (2.68 ± 0.14 nmol/g tissue, p < 0.01) compared to the control (halquinol), followed by the T4 group (3.68 ± 0.49 nmol/g tissue, p < 0.05). Furthermore, although the T2 (5.4 ± 0.99 nmol/g tissue) and T3 (5.85 ± 0.68 nmol/g tissue) groups also showed reduced MDA levels, these changes were not significant (p > 0.05).
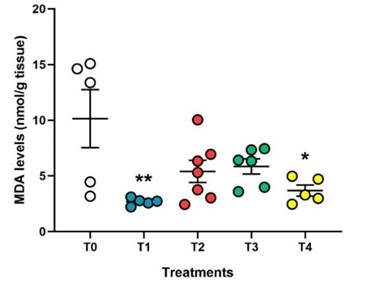
Figure 3 Effect of halquinol and nanoencapsulated EOs supple-mentation on oxidative stability of broiler chicken’s thigh meat. Control group: received a conventional diet supplemented with halquinol (60%). The experimental groups (T1-T4) received a diet supplemented with N-EOs from soursop (S), lemon (L) and eucalyptus (E) in different proportions (%): T1 (S:33.33%, L:33.33% and E:33.33%), T2 (S:50%, L:25% and E:25%), T3 (S:25%, L:50% and E:25%) and T4: (S:25%, L:25% and E:50%). Each circle represents an individual value, in addition the horizontal line is the average value, and the vertical line represents the standard error of the mean (n = 5). n.s = not significant, statistical differences: * P < 0,05; ** P < 0,01; *** P < 0,001 vs. control (halquinol).
The addition of nanoencapsulated EOs significantly reduced malondialdehyde (MDA) levels in chicken thigh meat, indicating a decrease in lipid peroxidation. Previous studies have reported the antioxidant properties of specific compounds present in EOs, such as eucalyptol in eucalyptus EO (Luís et al., 2016) and δ-cadinene, α-muurolene, β-caryophyllene, epic-α-cadinol, and α-cadinol in soursop EO (Gyesi et al., 2019; Kossouoh et al., 2007). These compounds are known for their radical scavenging and lipid peroxidation inhibition properties.
Table 4 Effect of halquinol and nanoencapsulated essential oils (N-EOs) supplementation on bromatological analysis and pH values of broiler chickens breast meat
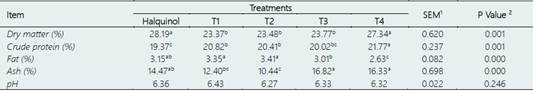
Treatments include - Control group: received a conventional diet supplemented with halquinol (60%). The experimental groups (T1-T4) received a diet supplemented with N-EOs from soursop (S), lemon (L) and eucalyptus (E) in different proportions (%): T1 (S:33.33%, L:33.33% and E:33.33%), T2 (S:50%, L:25% and E:25%), T3 (S:25%, L:50% and E:25%) and T4: (S:25%, L:25% and E:50%).
a,b,cMeans with different superscripts within columns differ significantly (P < 0.05).
1SEM, standard error of the means.
2P-values associated with dietary treatment.
The reduction in MDA levels observed in our study is consistent with the findings of previous studies that demonstrated the antioxidant effects of EO supplementation in broiler chickens (Mohebodini et al., 2021).
4. Conclusions
In conclusion, our findings indicate that the dietary supplementation of nanoencapsulated EO mixtures holds promise as a strategy to improve the quality of poultry meat. The supplementation of N-EOs resulted in improved growth performance, gut morphology, meat hardness, and bromatological parameters in broiler chickens. Additionally, the nanoencapsulated EOs exhibited antioxidant effects by reducing lipid peroxidation. These results support the potential use of N-EOs as an effective alternative to halquinol supplementation in broiler production. However, further research is needed to explore different variables, such as packaging and storage, and to gain a deeper understanding of the effects of N-EO mixtures in poultry production.