1. Introduction
Potato (Solanum tuberosum L.) is the third most important food crop in the world and plays a vital role in the global food system (Birch et al., 2012). Recent studies have emphasized that biotic and abiotic environmental stresses limit and reduce the production and productivity of food crops (Waqas et al., 2019; Gull et al., 2019). Factors that impact on potato yield are not well understood, however, maintaining yield under stress conditions is one of the highest focusses of modern agriculture (Campbell et al., 2022). For this reason, it is important to develop adequate protocols to measure the impact of these stresses on the yield of food crops, and even more so when the threat of climate change becomes increasingly important.
Potato production in Peru has been preparing for the impact of climate change. An INIA report published in 2012 indicated that, of a total of 300,000 hectares of potatoes sown annually in Peru, around 120,000 hectares corresponded to the clone INIA 303 - Canchán or simply Canchán, one of the most promising genotypes to face climate change, with a yield of up to 30 t/ha compared to the national average yield of 13.3 t/ha (INIA, 2012). Peru is the largest producer of potatoes in Latin America, with a production of around 5.3 million tons, distributed in 19 regions, mainly the Andean highlands where two groups of varieties, white and colored hybrids as well as some native ones are of commercial importance (MIDAGRI, 2018).
In recent years, studies on the influence of various physiological, genetic and microbiological factors on potato tuberization have been diverse. Thus, we have the studies on hormonal regulation and auxin-cytokinin signaling pathways in gene expression (Kolachevskaya et al., 2021), the use of supplements (Dalimunthe et al., 2021), the effect of red LED light and blue LED light, correlated with a greater expression of tuberization-related genes (Pundir et al., 2021), the use of a culture medium with a high content of sucrose and gelrite (Herrera-Isidron et al., 2021), and the application of microbiological substances (Fedorova et al., 2021). However, there are not many studies that relate potato tuberization to stress conditions, especially heat stress, hence the need for this study to fill a gap in our current knowledge.
Heat stress, due to climate change, affects various stages in the growth and development of plants, leading to severe losses in crop yield (Kaushal et al., 2016; Li et al., 2018). However, few studies on heat stress and even fewer studies have been carried out on potato (Gelmesa et al., 2017). A simple evaluation of the effect of heat stress on plants, under laboratory (in vitro) and field (ex vitro) conditions, is to measure biomass, plant height and root growth (Chaudhary et al., 2020). In potato, the most important characteristic to evaluate, under heat stress conditions is tuberization (Gopal et al., 1998). Studies on in vitro tuberization of potato showed differences in the number and weight of tubers and weight of plantlets, in all genotypes evaluated at both room temperature (18 °C) and elevated temperature (25 °C) (Singh et al., 2016). In an evaluation of 42 potato cultivars, using an in vitro tuber induction system, under heat stress (35 °C/28 °C, day/night) for 30 days, only three cultivars showed the highest tolerance to heat, while in the rest of the cultivars, although the plant height growth increased, damage was also observed in the cell membranes of chloroplasts, decrease in chlorophyll biosynthesis and photosynthetic efficiency (Zhang et al., 2024). On the other hand, in recent years, due to the phenomenon of climate change, plants are being used to study the combined effect of stresses such as high temperatures and drought. This is the case of potato, where transgenic potato plants overexpressing an ethylene response transcription factor (StERF94) were studied under in vitro culture conditions, observing that the StERF94 overexpression improved the tolerance of the transgenics to such stressful conditions (Charfeddini et al., 2023).
In the induction of in vitro potato tubers in a wide range of genotypes the incubation was at 22 °C (Estrada et al., 1986). In other cases, the tuberization phases were carried out at 21 °C (Garner & Blake, 1989) and 18±1 °C, observing that no tuberization occurred at 25±1 °C (López-Delgado & Scott, 1997). Likewise, the incubation was carried out at 24 °C (Fufa & Diro, 2014), 20 oC (Mamiya et al., 2020) and 18-20 °C (Mohamed & Girgis, 2023). However, using the temporary immersion bioreactor system in the evaluation of potato clones, the stressful conditions of high temperature (30 °C) made it possible to distinguish non-deformed tubers from deformed tubers, as well as the increase in the biomass of plant tissues, the number of tubers affected by heat stress and the weight in most of the clones evaluated (Gautam et al., 2021). These differences would be due to the genetic variability of the potato genotypes evaluated. But not only in studies on stress tolerance in potatoes under in vitro conditions have shoot apex or nodal segments been used. A recent study on the thermo-tolerant effect on cell suspension culture in potato, with a duration of stressful pretreatment at 5-50 °C for 20 min, it was observed that the viability of the cells was affected between 85 to 25% with heat stress between 35 and 50 °C, although cell viability during the recovery period, between 35 to 35 °C, increased up to 75% (Harun-Or-Rashid et al., 2024).
In vitro evaluation of genotypes against various biotic and abiotic factors offers several advantages, compared to conventional evaluation methods (Gautam et al., 2021). These advantages are as follows: quick evaluation of numerous genotypes in reduced space, low cost and strict control of environmental conditions; and both physical (e.g. temperature, photoperiod and osmolarity) and chemical (e.g. heavy metals, herbicides and salinity) properties, without the influence of the variable and complex nature of these stresses that manifest under greenhouse and field conditions that can significantly alter the results (Gautam et al., 2021; Pérez-Clemente & Gómez-Cadenas, 2012).
The causes of tolerance to heat stress have not been established, however we hypothesize that this tolerance is found in the genetic variability of potato genotypes, so in this study we aimed to investigate the effects of moderate heat stress on tuberization in nine potato genotypes, using an ex vitro and in vitro system.
2. Methodology
2.1. Ex vitro and in vitro plant material
The plant material studied under in vitro and ex vitro conditions (greenhouse) was constituted by nine genotypes of potato (S. tuberosum): Amarilis, Atlantic, Canchán, Capiro, Liberteña, Leona, Perricholi, Tacna and Yungay. These genotypes were selected because they are the most demanded and consumed in all regions of the country. This plant material (two plantlets per clone) was provided by the International Potato Center (CIP - Peru) as in vitro cultures, with the corresponding Phytosanitary Certificate indicating that they were obtained by meristem culture and were free of systemic diseases. Subsequently, the plantlets were multiplied in vitro many times to obtain enough plant material for the establishment of the trials.
2.2. Preparation and acclimatization of ex vitro plant material
Plants of 3-5 cm tall and with good development of the roots system were obtained after 2-3 months from in vitro plantlets propagated by culture of shoot tips and nodal segments. The plantlets were pre-acclimatized, under laboratory conditions, for 3-5 days and placed in small containers (15 mL-1) with sterilized distilled water in order to strengthen the root system. Subsequently, they were estab lished in greenhouse bags (100 g) with sterilized river sand substrate and humus in a 2:1 ratio. During the first 15 days, the seedlings were covered with transparent polyethylene cups and always kept moist to avoid dehydration. The plants were in an area of the greenhouse with about 70% shade. Irri gation was carried out daily with running water.
2.3. Tubers harvest ex vitro
The harvest of the tubers of 0.5 to 2.0 cm in diameter was carried out 3-4 months after the plantlets were established under greenhouse conditions and reached an average height of 30 cm.
2.4. Clonal propagation and establishment of in vitro tuber assays
Potato plantlets from both ex vitro and in vitro tests were successively propagated in in vitro conditions by shoot tips and nodal segments culture following the methodology established at the CIP (Tovar et al., 1985). All the culture media contained MS mineral salts (Murashige & Skoog, 1962) and the vitamins m-inositol 100 mg/L-1 and thiamin.HCl 1.0 mg/L-1. In the case of the in vitro tuberization tests, the initiation culture medium (first phase) incorporated sucrose 3.0%, the growth regulators 1-Naphthaleneacetic acid (NAA) 0.01 mg/L-1 and gibberellic acid (GA3) 0.25 mg/L-1 and agar-agar 0.7%. The multiplication culture medium (second phase) was modified by incorporating 2.0% sucrose and the growth regulators 6-Benzylaminopurine (BAP) 0.5 mg/L-1, NAA 0.01 mg/L-1 and GA3 0.4 mg/L-1. The tuber culture medium (third phase) was also modified with the incorporation of sucrose 8.0%, BAP 5.0 mg/L-1 and chlorocholine chloride (CCC) 500 mg/L-1 added as a plant growth retardant used in the in vitro induction of potato tubers, improving carbohydrate accumulation, and decreasing GA (Estrada et al., 1986; Garner & Blake, 1989). In the same glass container, the multiplication culture medium was removed and replaced by the tuber culture medium, so both culture media were kept liquid. In the case of ex vitro tuberization tests, only the initiation culture medium was used.

Figure 1 In vitro plantlets of Canchán genotype in multiplication culture medium after 9 months of culture. (a) Without tuber formation. (b) Forming a single tuber. Incubation temperature: 20 to 22 °C.
Table 1 Temperatures recorded during the initiation phases (in vitro) and ex vitro tuberization (greenhouse) of nine genotypes of potato (2019)
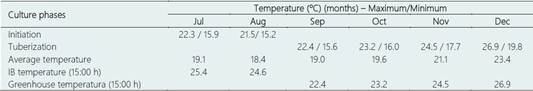
IB, Institute of Biotechnology. Only the temperatures of the IB (15:00 h) were recorded by the Coolbox device. The other temperature records were taken from the Lambayeque Meteorological Station.
Table 2 Temperatures recorded during the initiation, multiplication and in vitro tuberization phases of nine genotypes of potato under in vitro conditions (2021-2022)
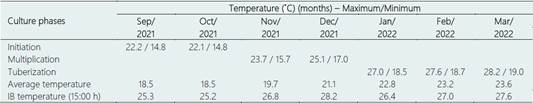
IB, Institute of Biotechnology. Only the temperatures of the IB (15:00 h) were recorded by the Coolbox device. The other temperature records were taken from the Lambayeque Meteorological Station.
In the multiplication phase, five explants were used, consisting of stem segments, without shoot apex, showing only 3-5 nodes per explant. In all cases, the pH of the culture medium was kept at 5.8 ± 0.1. During the initiation phase 18 x 150 mm test tubes were used, and transparent glass flasks of 11.5 x and 5.5 cm diameter, were used in the multiplication and tuberization phases.
Based on previous observations made in our laboratory and on preliminary results, the multiplication culture medium was used as control treatment under the same temperature conditions of the explants established in the tuberization culture medium, because under normal temperature conditions (20 to 22 °C) the formation of tubers was not observed until after 9 months of culture (Figure 1). Likewise, all the nine genotypes in tuberization culture medium under normal temperature conditions, formed tubers in a greater and lesser proportion (data not shown).
Incubation conditions
The incubation conditions of the cultures were those recorded in the greenhouse and incubation room of the laboratory of the Institute of Biotechnology (IB) of the Pedro Ruiz Gallo National University (Lambayeque, Peru). No type of cooling and/or ventilation equipment was used.
The incubation conditions of the ex vitro tests were as follows: variable temperature, in the initiation phase of the in vitro cultures in the IB (July and August/2019), and in the tuberization phase in the nursery (September to December/2019) (Table 1).
The incubation conditions of the in vitro tuberization tests in the IB, were the following: variable temperature in the initiation phase (September and October/2021), multiplication phase (November and December/2021), and tuberization phase (January to March/2022). The ambient temperature data was taken from the Lambayeque Meteorological Station (San José, Lambayeque), code 106108, Lat. 6º44’3.75”, Lon. 79º54'35.4" and 18 meters above sea level. The temperature in the IB was performed at 15:00 h, using a Coolbox indoor and outdoor thermo-hygrometer installed in the same place where the tests were established (Table 2).
Relative humidity was 85%, illumination 10 W.m-2, during the initiation and multiplication phases, and 1 W.m-2 during the tuberization phase, using daylight-type fluorescents and a photoperiod of 16/8 h (day/night).
Experimental design and evaluations
In in vitro system eight flasks per clone were used in the establishment of trials, but in order to standardize the trials only six non-contaminated flasks were evaluated. Three types of tuberous formations were established: the tubers themselves made up of round to slightly round tubers; elongated to totally deformed tubers; and thickened stolons. A randomized complete block design was employed in nine (9) genotypes, two (2) treatments, six (6) flasks and five (5) explants per experimental unit. The cul ture medium treatments tested were: Multiplication culture medium and tuberization culture medium. The evaluations of the tuberization process, both in ex vitro and in vitro conditions, were carried out after 120 and 90 days, respectively.
3. Results and discussion
As shown in Table 1, during the ex vitro tuberization system, specifically during the initiation phase (in vitro), all the genotypes studied reached optimal growth within the two months of the process (July and August/2019) where the average temperature recorded in the laboratory of the IB was 25.4 and 24.6 °C, respectively. This same table shows that the average temperature of tuberization (greenhouse) varied between 22.4 to 26.9 °C (September to December/2019) (Table 1).
The in vitro tuberization system shows the average temperature recorded in the IB laboratory ranged from 26.4 to 27.6 °C (January to March/2022) (Table 2). In the control treatment made up of the multiplication culture medium under the same temperature conditions, tuber formation was not observed in any of the genotypes tested.
The increase in potato production in the coming years in various regions of the world will go hand in hand with the release of higher-yielding varieties, greater resistance to pests and diseases, and greater tolerance to extreme stressful conditions. In the case of environmental factors, heat, cold, salinity drought, and flood are the main causes of adverse effects on the growth, development, and productivity of food crops (Tiwari et al., 2020; Lal et al., 2022). To these factors would be added the increase in the intense pressure of climate change, which would make it necessary to direct scientific research to combat the adverse effects of climate change and guarantee food and nutritional security for the coming decades (Gómez-Zavaglia et al., 2020).
3.1. Contamination
During in vitro tuberization tests, bacterial contam ination was observed to be the greatest difficulty in establishing the tests in the potato multiplication and tuberization phases (November/2021 to March/2022 with a temperature of 26.8 and 27.6 °C, respectively) (Table 2). This difficulty was greater in the Amarilis and Atlantic clones (around 50%) and to a lesser extent in the Capiro, Leona, Liberteña, Tacna and Yungay genotypes (around 25%).
In vitro contamination during the tuberization phase, had not been observed in in vitro cultures during the initiation phase.
Biological contamination in in vitro plant cell cultures is often a problem. In the case of the contamination of the potato culture observed in this study, permanent manipulation and the conditions of the laboratory should be considered for in vitro cultures which are strictly aseptic as they came from the International Potato Center (CIP). It is possible that the new culture conditions as the change in the consistency of the culture medium (gelled to liquid); the dramatic increase in sucrose (2.0% to 8.0%) and cytokinin BAP (up to 5.0 mg/L); the CCC supplement (500 mg/L); the conditions of almost total darkness (1 W.m-2); and high temperatures (>26 °C), have generated an optimal environment for the exponential multiplication of bacterial pathogens. However, in most in vitro potato cultures, even with visible microbial contamination, the plants reached optimal growth and development.
3.2. Ex vitro tuberization
Table 3 shows that among the nine potato genotypes evaluated, ex vitro tuberization occurred in seven of them (Amarilis, Atlantic, Canchán, Capiro, Perricholi, Tacna, and Yungay), except for the Leona and Liberteña clones, where no tubers formed. In the relation number of acclimatized plants/number of harvested plants the clones Amarilis (15 tubers formed) and Canchán (34 tubers formed) reached the highest ratio with 45.9% and 45.7%, respectively. However, other genotypes with a lower ratio such as Yungay (25.5%) and Atlantic (32.6%) surpassed the Amarilis clone with 21 tubers formed. The Canchán genotype surpassed the other genotypes with the total production of 34 (25.4%) tubers out of a total of 134 tubers produced. Likewise, the number of tubers formed/acclimated plants ratio was less than 1 in all the genotypes evaluated, highlighting the Canchán and Tacna genotypes with 0.97 and 0.62, respectively.
Out of the total number of tubers formed (134), most had a size between 5-10 mm (47.0%) followed by a size <5 mm (32.8%) and only 20.1% reached a size >10-20 mm (Figure 2). The Canchán genotype reached the highest number of tubers formed (34/25.4%). However, the majority had a size of less than 5 mm (19/43.2%). In the Atlantic and Capiro genotypes, sizes of 5-10 mm were mostly reached with 15/23.8% and 10/15.9%, respectively. In the Tacna genotype, the largest number of tubers (13/48.1%) had a size greater than 10-20 mm. Figure 3 shows the formation of tubers in some potato genotypes, as well as a tuber of the Canchán genotype of normal size.
In the study presented, in the ex vitro system the maximum tuberization temperature, recorded every day at 15:00 h from September/2019 to December/2019, were progressively ascending from 22.4 to 26.9 °C.
Table 3 Production of tubers of nine potato genotypes under ex vitro conditions (greenhouse) (September - December/2019)
| Genotype | Acclimatized plants (No) | Harvested plants with tubers (No/%) | Tubers/ Plants (No) | Tubers/ Total (No) | Ratio = Tubers formed/ Acclimatized plants |
|---|---|---|---|---|---|
| Amarilis | 37 | 17 (45.9) | 0-4 | 15 (11.2) | 0.41 |
| Atlantic | 43 | 14 (32.6) | 1-4 | 21 (15.7) | 0.49 |
| Canchán | 35 | 16 (45.7) | 0-4 | 34 (25.4) | 0.97 |
| Capiro | 50 | 11 (22.0) | 0-3 | 13 (9.7) | 0.26 |
| Leona | 33 | 0/0 | 0 | 0 | 0 |
| Liberteña | 42 | 0/0 | 0 | 0 | 0 |
| Perricholi | 51 | 18 (35.3) | 0-3 | 14 (10.4) | 0.27 |
| Tacna | 26 | 9 (34.6) | 1-4 | 16 (11.9) | 0.62 |
| Yungay | 51 | 13 (25.5) | 0-3 | 21 (15.7) | 0.41 |
| Total | 368 | 98 (26.6) | 134 |
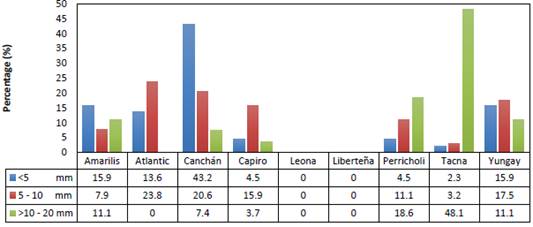
Figure 2 Size of tubers of nine potato genotypes under ex vitro conditions (greenhouse) (September - December/2019).
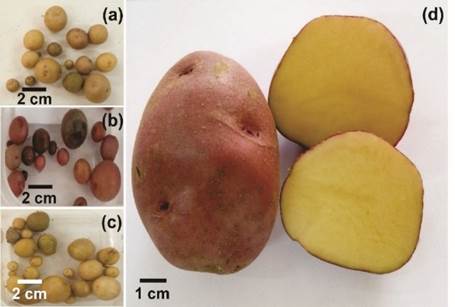
Figure 3 Examples of tubers of some potato genotypes. (a) Perricholi; (b) Canchán; (c) Yungay and (d). Canchán tuber of average size. (Maximum size 8.0 cm).
In this system, the production of potato tubers as a seed source, under greenhouse conditions was observed in the nine genotypes evaluated, with the total tuber production of < 5 mm (32.8%), 5-10 mm (47.0%) and > 10-20 mm (20.2%) in the period of 75 to120 days of growth in soil. Ahloowalia (1994) in a similar study reported that micropropagated plants produce tubers between 9 and 15 mm after 70-115 days of growth in soil, noting that tuber production is influenced by the explant number, duration in vitro culture and genotype. These results were very similar to those obtained in the study presented, where Canchán far exceeded the other genotypes evaluated, with the highest production of tubers between 90 to 120 days and the highest percentage of plants that formed tubers (45.7%), although with high production of tubers formed per acclimated plants (43.2%), reaching the minimum diameter < 5 mm.
Other studies have shown that potato plant growth is faster between 20-25 °C while the optimum range for tuberization and tuber growth is between 15-20 °C (Singh et al., 2015). It has also been reported that the potato develops best at about 20 °C and it has been postulated that the effect of high temperatures depends on the growth stage, since the earlier it occurs, the more negative its impact will be on the growth and yield of the potato (Rykaczewska, 2013; 2015). Likewise, tuber yield and dry matter partitioning is best with an optimum temperature of approximately 20 °C (Timlin et al., 2006); tuber induction at 15 °C, initiation at 22 °C and setting at 15 °C (Struik, 2007). For this reason, in the study presented, even when tuberization temperatures were moderately high (around 28 °C), they were detrimental for most of the nine potato genotypes evaluated, highlighting only the Canchán, Yungay and Atlantic genotypes. On the other hand, it is important to indicate that several authors have tested the effect of very high temperatures on potato plants such as 35 °C/25 °C (day/night) (Rykaczewska, 2015; Timlin et al., 2006; Struik, 2007; Chen & Setter, 2021), and high day/night temperature of 30.8 - 21.8 °C and 31.2 - 24.1 °C, during the tuber initiation and tuber bulking phases (Kim & Lee, 2019), observing in all cases a significant reduction in tuber production.
Regarding the ratio of number of tubers formed, there is only one study carried out under greenhouse and field conditions, where the temperature was optimal and the ratio ranged from 2.9 to 5.0 (Lal et al., 2022). This was a significantly high figure compared to what was reported in this study where only in Canchán the ratio between tuber formed/acclimatized plants was 0.97. On the other hand, all the tubers formed were mostly round to some slightly oblong. Also reported by Ahloowalia (1994), this may be due to internal physiological defects (Rykaczewska, 2015). All the tubers had well-formed "eyes" and after the withering period (1-3 months) the tubers began to sprout until they formed normal plants. In this regard, the impact of temperatures of 35 °C/25 °C day/night was evaluated in six varieties of potato. This was studied over a period of three growth periods in two consecutive years, which has shown that high temperatures in the later stages of plant development had a negative effect caused by the deformation of the tubers (elongated tubers, bottlenecks, second growth or chain tuberization and gemmations). This was greater depending on the stage of plant development (Rykaczewska, 2015). In the study that is presented here, the temperature affected the number of tubers formed, but it did not influence the occurrence of tuber deformations. This is possibly because between the months of September to December/2019, the minimum temperatures recorded at night were in a range from 15.6 to 19.8 °C.
3.3. In vitro tuberization
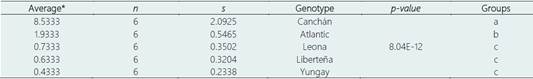
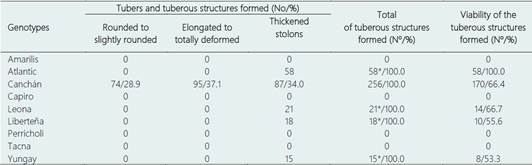
Of the nine potato genotypes tested, only in Canchán the formation of tubers and tuberous structures was observed in all the repetitions (glass flasks) evaluated. In Atlantic, Leona, Liberteña and Yungay, the formation of only thickened and slightly thickened stolons (50-100%) were observed, while for Amarilis, Capiro, Perricholi and Tacna no evidence of tuberization was observed (Table 4,5). Of the potato genotypes that showed tuberization, only Canchán induced the highest number of tubers, under the temperature conditions evaluated, followed by Atlantic. Leona, Liberteña and Yungay showed the least tuber formation. The analysis also showed that there were no differences between the blocks (flasks) (Table 4).
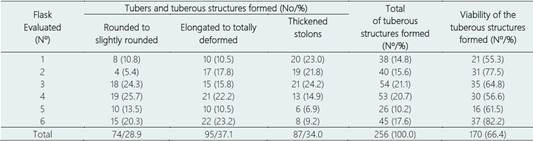
Table 4 Experimental design of randomized complete blocks in vitro tuberization of nine potato genotypes, under moderate heat stress conditions, in tuberization culture medium
*Total of tuberous structures formed (N°).
Table 5 In vitro tuberization (tubers) in nine potato genotypes under moderate heat stress conditions
*Only thickened stolons formed.

Figure 4 Responses of two potato genotypes to stressful conditions of moderate heat temperature, under in vitro cultures. (a). Canchán (tolerant) and (b) Yungay (not tolerant).
The plantlets of the genotypes that did not tuberize showed various morphological and physiological abnormalities such as thickening of stems and leaves, thickening and browning of roots, thinning, chlorosis and shoot fasciation (Table 5, Figure 4). However, once restored to a gradual light condition and temperature (20-22 °C), most genotypes started a new cycle of clonal propagation (re-growth).
In in vitro system (IB laboratory) the maximum tuberization temperature, also recorded at 15:00 h from December (2021) to March/2022, was de 28.2 to 27.6 °C. In this system, of the nine genotypes evaluated, only the Atlantic, Canchán, Leona, Liberteña and Yungay genotypes induced the formation of tubers, with the Canchán genotype standing out widely from the rest with the formation of 256 tubers with the following characteristics: rounded to slightly rounded (28.9%), elongated to totally deformed (37.1%) and thickened stolons (34.0%). In the Atlantic, Leona, Liberteña and Yungay genotypes only 58, 21, 18 and 15 tubers were induced and only in the thickened stolons form.
Various studies have indicated that under high temperature conditions (30/20 °C day/night) the biomass of potato plants (without tubers) increased significantly due to the migration of photosynthates towards the foliage (Basu & Minhas, 1991; Hancock et al., 2014). As already indicated, in the study of Rykaczewska (2015) and Chen & Setter (2021), working under temperature conditions of 35/25 °C (day/night), they concluded that the total yield should not be the only indicator of the tolerance to heat stress but also secondary tuberization and physiological defects of tubers. Likewise, similar results were also observed in potato microtuberization using the temporary immersion bioreactor system under heat stress conditions (30 °C), where the number and weight of tubers varied among the genotypes evaluated (Gautam et al., 2021). These results correlate with those obtained in the study presented here where not only tuberization was recorded in five of the genotypes evaluated but also with the numerous tubers elongated to totally deformed and thickened stolons formation.
Physiological deformations such as elongated tubers, bottlenecks, secondary growth (chain tuberization) and budding were observed under heat stressful conditions (35/25 °C (day/night) in greenhouse and field (Rykaczewska, 2015), and at 30 °C deformed tubers with sprouts and deformities with excessive excrescences and unusual shapes (Gautam et al., 2021). Most of these forms have been observed in the study presented here, partially in Canchán and absolutely in the Atlantic, Leona, Liberteña and Yungay genotypes.
Regarding the influence of environmental incubation factors, in addition to temperature, such as photoperiod and lighting, it is important to note that in the study by Estrada et al. (1986) evaluated tuberization in vitro in around 50 potato genotypes, mostly tetraploid, under conditions of 22 °C and total darkness, observing tuberization in all genotypes after 40 days of culture. In the study presented here, the lighting condition was only 1 W.m-2, one tenth of the lighting used in the initiation and multiplication phases, so it is possible that the effect of the photoperiod would be attenuated by the minimum lighting used.

Figure 5 Formation of in vitro tubers of the Canchán potato genotype under moderate heat stress conditions. (a). Non-deformed tubers; (b). Deformed tubers; (c). Thickened stolons. All these structures can be micropropagated.
3.4. In vitro tuberization in Canchán genotype
The Canchán genotype was the only one where 100% formation of tuberous structures was observed (rounded to slightly rounded tubers, elongated to totally deformed tubers and thickened stolons) (Table 6, Figure 5). Of the total tuberous structures formed (256), the largest number corresponded to elongated and totally deformed tuberous structures (95/37.1%) followed by thickened stolons (87/34.0%) and rounded to slightly rounded tubers (74/28.9%). The range of variation in the number of tuberous structures formed was very wide, from as low as 26 (10.2%) to as high as 54 (21.1%).
The viability (re-growth) of the tuberous structures formed by Canchán in the replications evaluated reached a range of 55.3% to 82.2% (Table 6). The differences corresponded to non-viable and contaminated tuberous structures.
It has been postulated that the genotypes that present deformed tubers under heat stress conditions should be considered as susceptible (Gautam et al., 2021). Canchán produces 29.69% of non-deformed tubers that can be used directly as seeds, while the remaining 37.20% of elongated to totally deformed tubers and 33.11% of thickened stolons, have high viability and can be used as material of micropropagation. Therefore, the evaluation and selection of potato genotypes Canchán is of primary importance to cultivation in regions of the world where global climate change has an extremely negative impact. Additionally, when this variety was released was only thought to be tolerant to frost and with a resistance to blight (INIA, 2012), therefore, it is the first time that tolerance to moderate heat stress has been evaluated.
The origin of Canchán is the result of joint collaborative research between the International Potato Center (CIP) and the National Institute of Agrarian Innovation-INIA. The commercial Canchán genotype was released in 1990 for possessing various agronomic and phytopathological attributes (INIA, 2012) such as earliness, high yield potential, red-colored tubers and white pulp, 25% dry matter content, frost tolerance and most relevantly, resistance to blight (Phytophtora infestans), a disease that cause the greatest loss in potato cultivation (Estrada et al., 1986).
4. Conclusions
This study has shown that the nine potato genotypes investigated in the tuberization process are affected differently by moderate heat stress conditions under both ex vitro and in vitro environments. This result suggests further evidence that temperature is one of the main factors that affect the growth and development of tubers, since in four evaluated genotypes no tuberization was observed, in another four genotypes scarce formation of thickened stolons and only in Canchán it was observed that 70% of the tuberization corresponded to tubers elongated to totally deformed and thickened stolons. No tuberization was observed in control treatments. Therefore, it is necessary to continue conducting studies on the Canchán genotype, of high commercial importance in Peru and other potato genotypes, evaluating other stressful conditions, such as drought stress, the induction of tubers in higher temperature ranges, the combined effect of photoperiod - stressful conditions and even the supplementation of other tuberizing substances such as acetylsalicylic acid and paclobutrazol. All this will contribute significantly to face the threat of climate change and contribute in some way to food security.