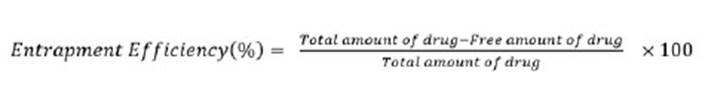INTRODUCTION
Today, significant advances have been made in the field of treating various types of diseases by utilizing modern techniques in drug delivery systems, which one of the most important is the use of nanotechnology. The advantage of using nanoparticles as drug delivery carrier is due to two properties of these materials:
First, nanoparticles, due to their small size, can penetrate through the very small capillaries within the cells, resulting in efficient drug accumulation in target areas in body. Another is that the use of biodegradable materials in nanoparticles produces a stable and uniform distribution of the drug at the target site for a period of several days or several weeks. In traditional Iran, the use of herbs and herbs for the treatment of various diseases has a long history. Due to the biological balance between the active ingredients in herbal medicines and other substances, these materials do not accumulate and do not cause side effects. Thus, they have a significant advantage compared to chemical drugs. One of the most widely used effective plants, native plant is sage(salvia officinalis L). Sage disposable medical history dates back to about 2000 years ago. The phytoestrogens in this plant have a significant role in the treatment of menopausal complications, especially hot flashes(1,2).
Across the globe is used to prevent menopausal symptoms, especially hot flushes from hormone replacement therapy (HRT) with estrogen alone or a combination of estrogen and progesterone(3,4). Because of the possible consequences of estrogen therapy increased the risk of breast cancer, endometrial cancer, vascular disease and stroke acceptance of this type of treatment is very low among women(5,6).
And only 3 to 8 percent of postmenopausal women are willing to accept treatment by hormone replacement therapy(7,8,9,10). In recent years, the use of nanoparticles in drug delivery systems has been very much considered. Because they have the ability to transfer medication to various parts of the body at regular intervals. In fact, the new features of nanoparticles provide the possibility of interacting with complex cellular structures.
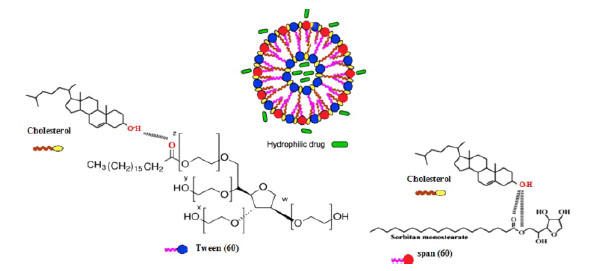
With the help of nanotechnology, targeted drug delivery can be achieved and controlled by the timing and place and speed of drug release. Among the various carriers, the vesicular systems for drug delivery, especially niosome due to their high capacity for drug entrapment, high storage time, drug release control, ease of preparation, correction of the target's various levels, the delivery of hydrophobic and hydrophilic drugs, Biodegradable and non-toxic, are considered as one of the best systems for studying the medication transfer. In studies conducted with marking of nanoparticles containing an anti-cancer drug using polyoxyethylene, the amount of trapping of niosome by the spleen and liver system can be greatly reduced(11,12,13). Varsha and colleagues developed a nano-capsule containing a mixture of curcumin and two anti-cancer drugs were doxorubicin(14,15).
Considering the effective role of Salvia officinalis in reducing hot flushes in postmenopausal women, this study investigated the effectiveness of niosome nanoparticles as carriers of (Salvia officinalis L) extract and a review of the sage extract release process.
MATERIAL AND METHODS
Span 60 (Merck), Tween 60 (Merck), cholesterol (Merck), chloroform %98, phosphoric acid %85, ethanol %96, (EDTA), sodium bicarbonate, sodium hydroxide %99, deionised water, Dialysis bag.
Digital scale was used with Japan's FX-60 model to weigh raw material.
Herdolph heater (D91126 Schwabach) was used to heat and stir the solutions continuously and uniformly
Fourier transform infrared spectrometer manufactured by Bruker Tensor 27 models in Germany to study the structure of raw materials and interaction of nanoparticles mid-infrared range 4000-400) cm-1).
The UV-Vis (Double Beam) model of the M350 manufactured by Camspec. The device is designed for transparent liquid spectroscopy and is equipped with a tungsten halogen bulb for visible light and a Deuterium lamp for UV light. The device is usable in the wavelength range of 160-700 nm.
The nucleus magnetic resonance imaging (NMR) with Bruker model was used to ensure the matching of chemical structure of the sage extract from the plant and the standard sample prepared from Yellowbend, in the presence of DMSO solvent.
The field emission scanning electron microscope with ( MIRA3TESCAN-XMU) model and the accelerator voltage 15 kv was used to investigate the morphology and size of synthesized niosome nanoparticles.
The Temperature control centrifuges device is made in England to isolate particles from a solution in which they float or to separate two liquid with different densities using centrifugal force.
Ultrasonic bath equipped with a thermostat with a frequency of 38 kHz, placing 110 watt (Kerry pulsatron) model made in England were used to reduce the size of nanoparticles synthesized vesicles.
oven with the (Wiseven- WoF-105) model, was used to remove the remaining chloroform solvent in niozum for 4 hours and 60 ° C.
The temperature control vibrator was used with the Wiseclean, Korea model to provide drug release conditions.
Rotary evaporator device with the (RE-47) Tokyo Japan model was used to evaporate chloroform solvent
Mechanical stirrer: In order to smooth and reduce the size of synthesized nanoparticles, mechanical boost was used with the DAIHAN Scientific, Korea model at speeds of 1,000,000.
Preparation of the microwave sage (salvia officinalis L) extract
In this method, the amount of 1 g of of Salvi leaves dried powder and using glass vial containing ethanol of solution %30 v/v placed in microwave (Milestone) for 9 minutes at 80 ° C with power of 500 watts. After the microwave extraction process, the obtained extract is pale yellow and is filter with a wattman paper and the product is stored for the next steps in a glass protected with an outer aluminum coating at -18 ° C(16,17,18).
Preparation of Niosome nanoparticles
Thin film hydration method was used to prepare niosome with cholesterol and without cholesterol(19,20). In this way, certain values of span 60, Tween 60, and if required, Cholesterol were weighted and dissolved in a 10 cc solvent of chloroform 98% in a , round-bottom flask . Then the solvent was removed using a rotary evaporator (50 rpm, 60 oc). At this stage, a milky oily thin film is formed around the flask wall. In niosomes with cholesterol, the oily film formed in the container wall is more solid and white While in niosomes without cholesterol the composed oily film is more fluid and milky color. In this case, round-bottom flask is containing niosomes synthesized by micro and a multi-layered vesicles (MLV). To ensure Lack of chloroform solvent , samples were placed for 4-8 hours in the vacuum oven. Then the samples were hydrated with the addition of double-water. In this step, to reduce size of nano particles of Niosome and convert the multilayered vesicles (MLV) into single-layer vesicles (SLV). The round-bottom flask were placed in the ultrasonic bath at a specified temperature and time, and mechanically agitated (1000 rpm). At the end , synthesized Niosome- nanoparticles were kept inside glass vials protected with aluminum paper to protect the light at 4 ° C(21,22,23).
Preparation of Niosome Containing loaded Extract
For the synthesis of niosome nanoparticles containing the sage extract, a significant amount of Span 60, Tween60, and cholesterol (for the preparation niosomes with cholesterol were weighed, and was poured into a round-bottom flask. Then, the sage extract 1% to 3% (w/w) ratio is added to the mixture of surfactants and cholesterol and is poured 10 ml of the chloroform on the final mixture. To make the appropriate interaction between the drug and the mixture of surfactants and cholesterol, the container containing the final mixture on a magnetic stirrer for 45 minutes placed to obtain a homogeneous solution. Then the container containing the sample is placed in the hot water bath-room at 60° C and the chloroform solvent is removed under vacuum conditions by a rotating evaporator. In this case, the niosomes are formed in the form of multilayer vesicles containing the drug as a thin film on the flask wall. Then, the flask containing Niosome and the drug loaded for 4 to 8 hours were transferred to vacuum oven at 60 ° C to emit additional solvent out , completely . For Niosoeme hydration step, 10 ml phosphate buffer pH=7/4 at 60° C was used(24,25). In the synthesis phase of the niosome nanoparticles containing the drug to reduce the particle size and the formation of single-layer niosomes, The sample container was placed in an ultrasonic bath at 60 ° C for 1 hour. The use of a mechanical stirrer is not necessary at this step and its use makes it difficult to interfere with the proper interactions between the drug and the mixture of surfactants and cholesterol in the encapsulation step. At the end , the niosome solution with the loaded drug , to continue the study, were kept inside glass vials protected with aluminum paper to protect the light at 4 °C(26) .
Determine entrapment efficiency of drug in the niosome nanoparticles containing the drug
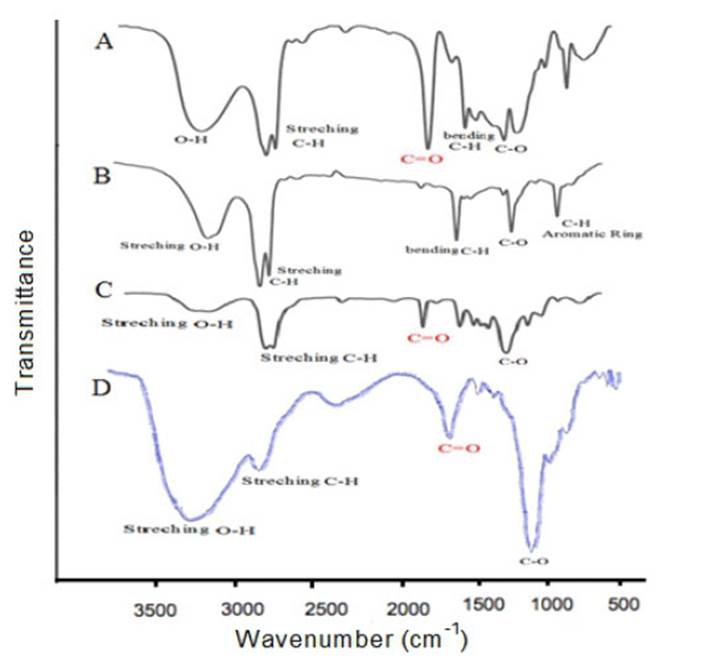
To determine entrapment efficiency sage extract loading in Niosome nanoparticles, we use Centrifuge technique. For this purpose, 6 samples of niosome with cholesterol and without cholesterol, containing loaded drug were transferred to the microtubules at three concentrations of drug and were placed under temperature control Centrifuges with a temperature of 4 ° C and speed of 13000 rpm for 40 minutes. The supernatant solution contains niosome, free drug and does not react to the drug. By measuring the supernatant absorption by the (UV-VIS) spectroscopy over a wavelength of 210 nm and by using the absorption numbers placed in the calibration equation, concentration of free drug was calculated. By having the initial drug concentration and the difference between these two values, the amount of the entrapment drug was obtained Eq1.
Evaluation of Drug Release from Niosome Nano carrier In vitro.
Dialysis bag technique was used to investigate process of release of extract from 3 Niosome with cholesterol and 3 Niosome without cholesterol containing loaded extract. After preparing the dialysis bags and transfer the solutions into it Samples were placed in erlenmeyer flasks -containing at phosphate buffer pH = 7.4 and placed at 37° C in incubator to have solution Condition of same to the body. Gradually, the loaded drug is released from niosome nanocarrier and over time, the concentration of the drug increases in the phosphate buffer. To measure the concentration of drug in the phosphate buffer during release, At specified intervals, each time, 3 ml of phosphate buffer are sampled and Its absorption is measured by (UV-VIS) spectroscopy over a wavelength of 210 nm. The concentration of free extract at the phosphate buffer is obtained using the calibration curve equation. After each sampling, 3 ml of the buffer is added to the environment, so that the sampling conditions are identical in all stages. The release process was reviewed within 5 consecutive days of the mentioned conditions.
Statistical Method
In this study, we used a non-parametric statistical method to study the results of drug release from three different weights of sage extract. Non-parametric methods are used to check data that is not naturally distributed and are much simpler in terms of calculation. In this statistical method, the common criterion for data dispersion (alternative to standard deviation) is the intermediate quartile. Median, divides the measurements samples into two equal parts. If each of these halves is again divided into two parts, the division points are called upper and lower quarks. The Interquartile range is not widely used in analytic work, but different statistical tests can be performed on it. Box-and-whisker plot, Sign test, Wilcoxon signed rank test are among the most used tests in non-parametric methods.
RESULTS
Characteristics results of Salvia officinalis L. extract by (NMR) spectroscopy
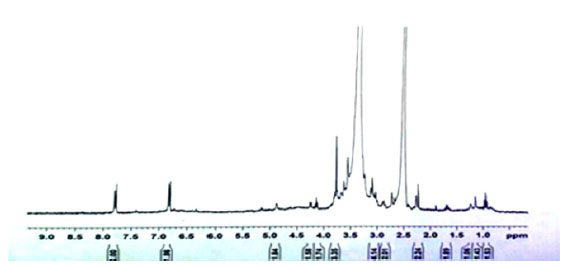
After preparing the microwave extract from the sage powder, the magnetic resonance (NMR) spectroscopy was used to identify the compounds in the extract and NMR spectra of standard ethanolic extract and ethanolic extracts was taken by microwave from sage powder in the presence of dimethyl sulfoxide solution (DMSO). As shown Fig (1) and (2).
The microwave extract is not filtered, so impurities are visible in Background of the spectrum. But the overall shape of the NMR spectra of both extracts is similar to each other. The appearance of this similarity indicates structural similarity of the compounds in both extracts. Therefore, we can safely use the microwave extract to load in synthetic nanocarrier.
FT-IR Characteristic Results of the Raw Materials Used and Synthesized Niosome
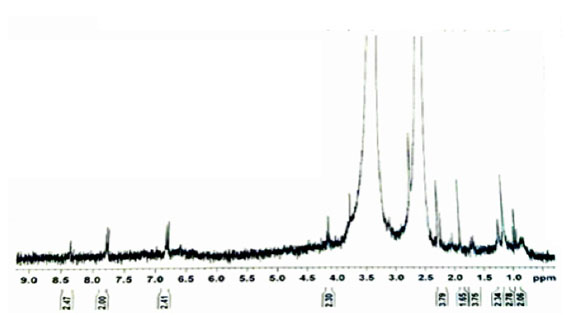
In Figure (3), we compare FT-IR spectrum synthetic niosome with Span 60, Tween 60, and cholesterol used in the synthesis. Fig (A) shows the FT-IR spectrum of the Span 60 surfactant. In this spectrum, the absorption bands in 1180 cm-1 refers to the stretching vibration of C-O bond. The absorption band in 1465 cm-1 refers to the bending vibration of the C-H bonds and the two messages in the range of 2854 cm-1 and 2920 cm-1 are associated with the symmetric and asymmetric stretching vibrations of the C-H bond of available sp3 carbon in the structure. The strong absorbance band in 1737 cm-1 is related to the vibration of the ester carbonyl group in the structure. The observed signal in 3392 cm-1 indicates the presence of the O-H group at the surfactant structure. Figure (B) shows the FT-IR spectrum of cholesterol. In this spectrum, we specifiy two peak in the region of 2853 cm-1 and 2919 cm-1 refered to the symmetric and asymmetric stretching vibrations of the C-H bond of available sp3 carbon in the structure. The message in 1468 cm-1 refers to the bending vibrations of the C-H bond. The absorption band in the range of 725 cm-1 indicates the vibrations of the C-H bonds of the aromatic rings of the structure. The specified peak in the 1122 cm-1 region is related to the vibrations of the C-O bond. In the cholesterol molecule, there is also a broadband in the 13302 cm-1 region, which indicates the vibrations associated with the O-H bond in the molecule. Figure (C) shows the FTIR spectrum of the Tween 60 surfactant. In this spectrum, the absorption band in the 1110 cm-1 region is related to the vibrations of the C-O bond in the molecule. The absorption band in the 1735 cm-1 is related to vibration of the ester carbonyl group in the structure. In Tween 60 molecule , two vibrations in the region of 2865 cm-1 and 2920 cm-1 are related to the symmetric and asymmetric stretching vibrations of the C-H bond of available sp3 carbon in the structure. The absorption band in the 3353 cm-1 region refers to the presence of O-H vibrations in the molecule. Figure (D) shows the FTIR spectrum of synthesized niosome. In the niosome, a strong absorbance band in 1104 cm-1 of the C-O bond vibrations and the absorbance band in 2927 cm-1 is related to the vibrations of C-H bonds in sp3 carbons. In the Niosome spectrum , the absorption band of the vibrations of the ester carbonyl group shifts to 1654 cm-1 Which is due to the strong hydrogen bond that exists between the carbonyl groups present in Span 60 and Tween 60 with the hydroxyl group in cholesterol molecule. Also, the absorption peak of 1338 cm-1 is related to the stretching vibration of the O-H group, which is wider due to the participation of the O-H group in the cholesterol and water molecule added in the hydration step.
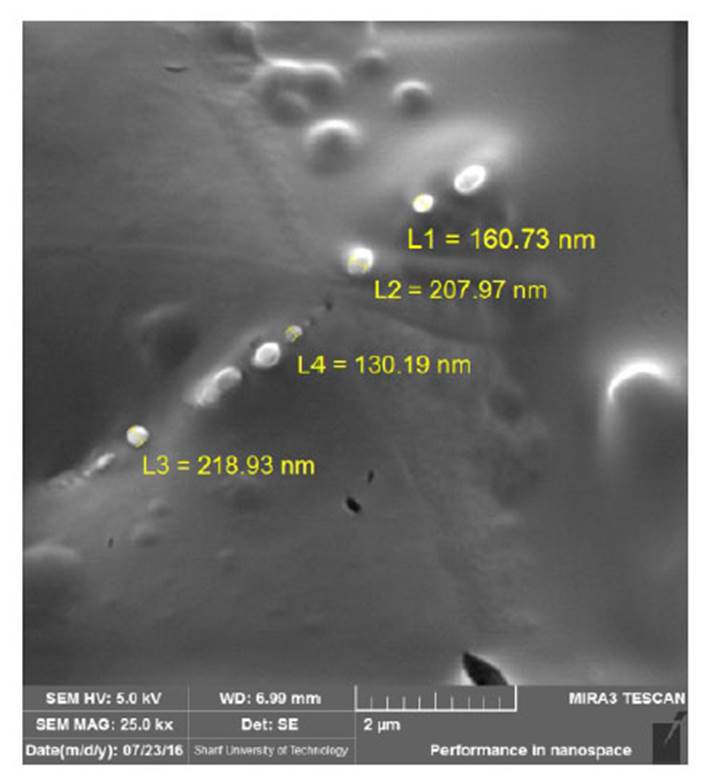
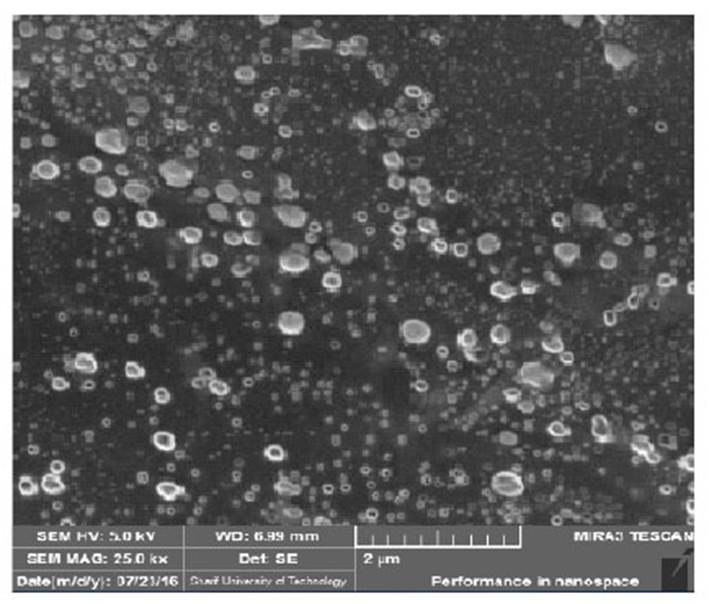
Results of FE-SEM Analysis of Niosome Nanoparticles With
To determine the morphology of niosome nanoparticles with cholesterol and without cholesterol , from samples, picture is taken by field emission scanning electron microscope. As shown in Fig (4 5), the size of the nanoparticles synthesized is within the range of 100-250 nm, and the particles are completely spherical. Due to the strong hydrogen bonding between the carbonyl groups in Span 60 and Tween 60 with the hydroxyl group in the cholesterol molecule, niosomes with Cholesterol are larger and uniform in size than niosomes without cholesterol.
Measurement of Zeta Potential
After determining the nano particles size with cholesterol and without cholesterol, the zeta potential was measured to evaluate its stability. This potential was achieved for niosome with cholesterol and without cholesterol -24/1 mv and 6/15- mv, Respectively. This indicates that niosome with cholesterol is more stable than harmful phenomena such as the accumulation and absorption of surfactants and the loaded drug leak compared to niosome without cholesterol.
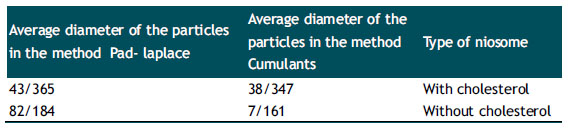
Results of DLS Analysis of Synthesis of Niosome Nanoparticles.
To determine the diameter of the particles containing niosome with cholesterol and niosome without cholesterol suspensions, we use Dynamic light scattering (DLS) Technique. This technique responds to the smallest variations in particle size due to the effect of light scattering. As expected, the mean diameters of niosome nanoparticles with cholesterol are greater than niosome without cholesterol (Table 1).
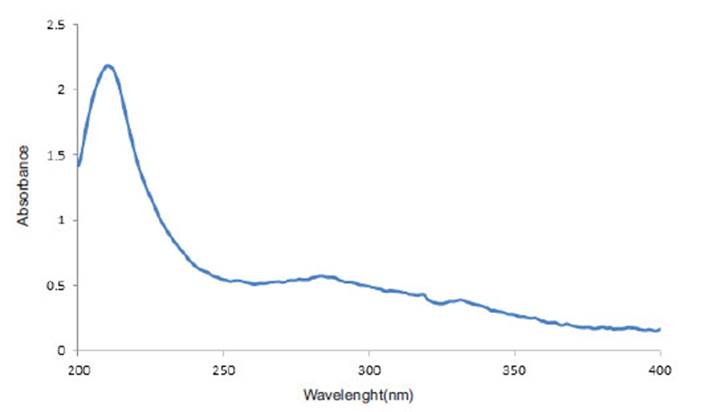
Investigation of The Interaction of Niosum Nanoparticles With Sage Extract by UV-Vis Spectroscopy.
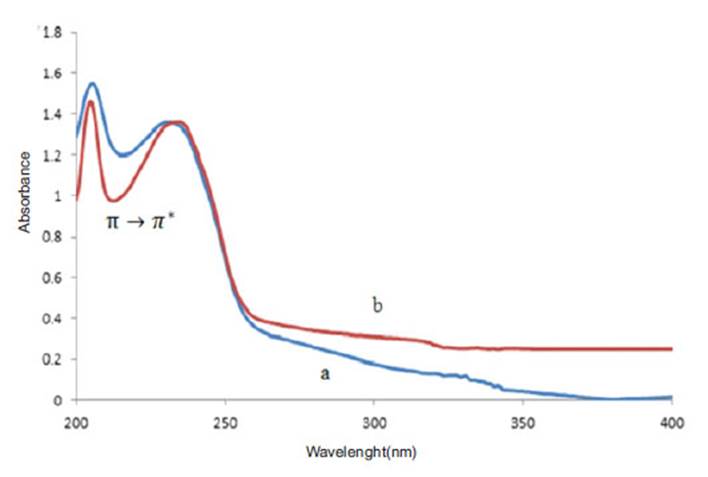
In order to investigate the interaction of sage extract and Niosome nano particles, (Uv-Vis) spectrum of sage extract , Niosome nanoparticles and nanoparticles containing loaded drug were prepared. As you can see in Figure (6), the sage extract has a maximum absorbance of 210 nm. Niosome with Cholesterol and without cholesterol also contain two peaks at 205 nm and another 231 nm. As shown in fig (7), the absorption intensity is reduced in the niosome containing the sage extract. Since the sage extract is a polar and solvent used for the hydration phase coating of phosphorus buffer with pH =7.4 , the inner environment of niosome has become more polarized and the transitions π o π * are directed toward longer wavelengths and this displacement towards higher wavelengths shows that the extract with niosome nanocarrier has a good interaction and transitions intensity π o π * increase in the nanocarrier containing the extract.
Analysing the Chemical Interactions Between the Nanocarrier and the Sage Extract
As shown in Fig (8), in niosomes with cholesterol, between ester carbonyl groups in the Span 60 and Tween 60 with the hydroxyl group (OH) in cholesterol molecule, Is created a strong hydrogen bond until bilayer structure of niosome is formed. Since the extracted sage is polarized, it is loaded inside the core and outer surface of the bilayer membrane, which are the aqueous parts of Niosome.
Analytical Parameters, Grading Function and Plot Calibration Curve of Standard Sage Extract
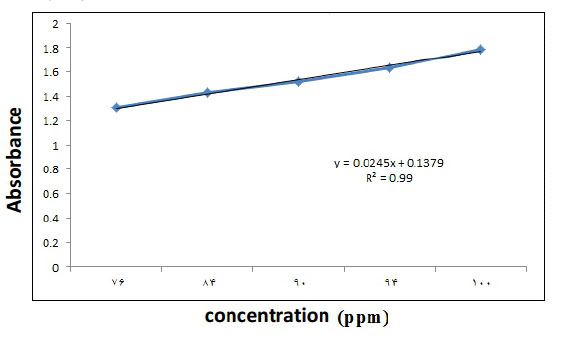
In order to determine the proper concentration of the drug that can be loaded onto the nanocarrier and the kinetics of its drug absorption and release process, it is necessary to plot the calibration curve of the drug in different concentrations. For this purpose, different concentrations of sage extract were prepared in the range of 76 ppm to 100 ppm. Using (Uv- Vis) spectroscopy solution absorption was read at a maximum wavelength of 210 nm, and the calibration curve and its lineary equation were obtained.
The obtained curve equation is y = 0.0245 x + 0.1379 , where y is the output signal of the device and x is the sample concentration in ppm. The correlation coefficient was 0.99, which indicates a good and meaningful linear relationship between the various concentrations of the extract of the relevant absorption signals.
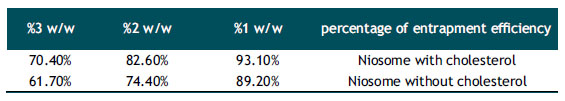
Determination of the Entrapment Efficiency for Sage Extract.
The results of determing the entrapmet efficiency sage extract from 6 samples of niosome nanoparticles with.
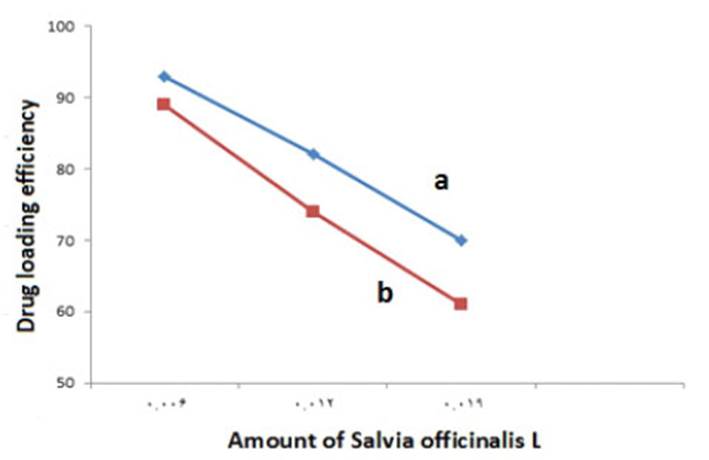
The Effect of the Amount of Drug on the Entrapment Efficiency.
The results of the effect of the amount of drug on the entrapment efficiency of the sage extract in niosome with cholesterol and without cholesterol are shown in Fig (10). As shown in Fig (10), with the increase in the amount of drug, the entrapment efficiency of the extract in both types of niosomes is reduced.
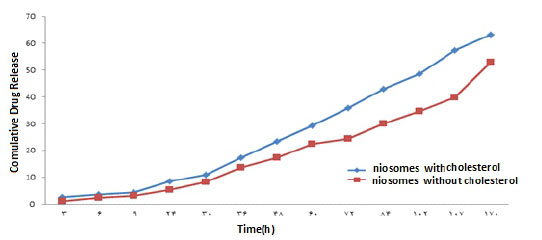
Evaluation of the Drug Release Rate of Niosome Nanoparticles.
The results of the study of drug release from the niosome with cholesterol and without cholesterol in the concentration range of %1 -%3 w/w of the extract in Figures (11, 12, 13) show that niosomes with Cholesterol of drug release in the first three days with a higher order and concentration than niosomes without cholesterol, and Sudden changes in the drug concentration due to the release of the drug appear to be More ideal and minimal. Also, a 1% w/w solution of niosome and Contained cholesterol has the highest drug release Percent than the other solution.
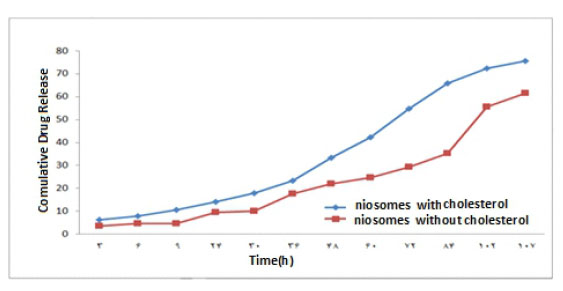
Figure 11 The Curve of the Drug Release of 1% W / W from Nano carrier in Phosphate Buffer With pH = 7.4
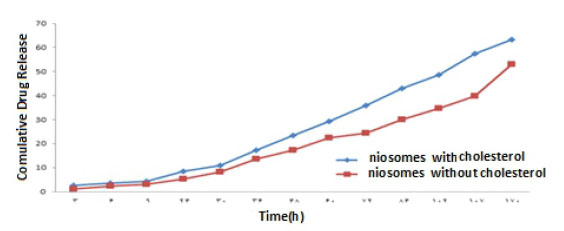
Figure 12 The Curve of the Drug Release of 2% W / W from Nano carrier in Phosphate Buffer With pH = 7.4
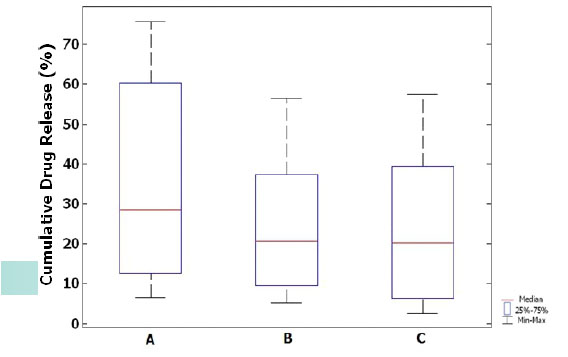
Interpretation of the Results of Drug Release by Plotting Box Plot Charts.
Box plot charts for drug release for three different drug concentrations from sage extract have been plotted in niosomes with cholesterol. The height of the boxes indicates that the release rate of the drug from Niosomes containing 1% w/w of the sage extract is greater than 2% and 3%. The height of the boxes shows that the results of the serial A (1%) simples are different with the serial B (2%) and C (3%) samples. The median data in Figures B and C show that data scattering is less and data distribution is normal than A. There are no outliers in any of the charts Fig 14.
RESULTS
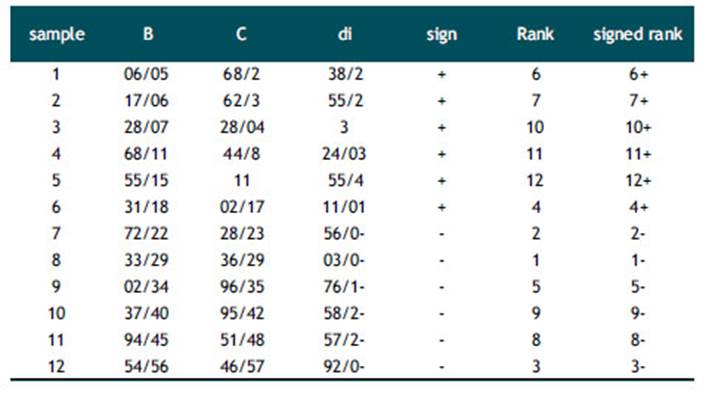
Clearly, according to the box plot, the results of the series A (1%) are different with those of the B series (2%) and C (3%). Therefore, the Wilcoxon Signed rank test (also known as Wilcoxon t-test) was used in two sets of samples B and C to evaluate the significance of the difference between the results of different concentrations. The two-way test was performed and the results were compared. The results of the Wilcoxon Signed rank test for the two C and B samples are presented in Tables (2,3,4). For each sample, the difference between the marks is taken between samples B and C. The value of the absolute value di, the difference between each pair of measurements is arranged. In the case of repetitive observations (nodes), the average of the rank values is replaced that there is no recurring view here. Thereafter, each rank is assigned the same sign with the sign of the difference. If there is no real difference between the two pairs sample , So there is not much difference between the sum of positive ratings (T+) and negative ratings (T-). In this test, we need to consider a zero assumption, and we examine the relationship, the effect, or the difference between the variables. Given a zero assumption, there was no significant difference between the results of B and C. If the value r = min (T+, T-) is less than or equal to the critical value obtained from the calculations in the table. In this work, the critical value for a two-way test at α =0/05 and n=12 is equal to 13. By knowing T+ = 50 and T- = 28, We can obtaine r=28. Therefore, zero assumption was accepted and there was no significant difference between the results of B and C. As a result, it can be said that the release of drug at different time points was the same for samples C (3%) and B (2%) and the concentration of extract did not have a significant effect on the release rate of the drug.
The studies carried out in this project show that the percentage of drug loading in niosomes with cholesterol is higher than from niosomes without cholesterol. The existence of a cholesterol molecule in a bilayer wall of niosome with cholesterol and the formation of strong hydrogen bonds between the hydroxyl group in the cholesterol molecule and the carbonyl groups present in Span 60 and Tween 60 makes the drug release from the niosome more controlled more uniform and more uniform slope. The highest loading and release efficiency of the extract was for the sample with a concentration of 1% w/w of niosome and with cholesterol. Therefore, the most suitable concentration for loading and release of the sage extract, was 1% w/w of sage extract. As a result, we can say that niosome is an effective and efficient nanocarrier to loading the hydrophilic extract of the sage