INTRODUCTION
Nowadays antimicrobial resistance represents one of the main global health issues 1. Which is defined as a “phenomenon whereby a microorganism is no longer affected by an antimicrobial to which it was previously sensitive 2), as a consequence of irrational use of antibiotics and wrong dosage 2,3. According to the World Health Organization (WHO), it has been estimated that between the years 2014 and 2016 one million people died from this phenomenon, and that by 2050 10 million people will die 4 5. Another global health problem is Urinary Tract Infections (UTI), being the second most frequent cause of an infectious disease in the female population between 18 and 24 years old 6,7. Meanwhile, they predominate in the male population in the first year of life and in those older than 50 years, due to prostatic pathologies and a wrongful manipulation of the urinary tract 8.
The Enterobactericeae family englobes the main causative agents as Klebsiella spp, Proteus spp y Escherichia coli9, the latter is the most prevalent organism both locally and globally 10, and is in the first category of "critical priority" of the WHO for presenting greater resistance 11. A study made in Spain showed that the most common multidrug resistant uropathogens were Escherichia coli and Klebsiella spp, which showed a higher resistance pattern in hospitalized patients 12. In our country in 2014, high resistance to Escherichia coli was reported, as in the Andean subregion 13. There was variation in which age group has the highest prevalence of antibiotic resistance. A study performed in Canada and the US in 2005 found that the age group with highest antimicrobial resistance in UTI’s where those over 65 years old 14. Likewise, another Spanish study carried out in 2011 reported that those over 60 years of age had greater antimicrobial resistance in the same disease 15. There is no consensus that establishes a defined age group, but there seems to be a vulnerability in the elderly group.
In 2012, the last report about antimicrobial resistance in Peru showed that Escherichia coli was the most frequent isolated bacteria at the hospital level, presenting a resistance to ampicillin greater than 80% and to nalidixic acid greater than 60% 16. Likewise, one of the most recent studies made in 2014, done by ORAS-CONHU with data from the Andean subregion concluded that the information in our country is scarce due to the absence of national surveillance 13. Even though the “Instituto Nacional de Salud del Perú (INS)” implemented a national antimicrobial resistance surveillance program, there is no recent data, therefore research in this filed remains pending and forgotten.
National information on antimicrobial resistance is quite limited and unclear, this data absence grows in high-andean regions, as is the case in the department of Puno, where there are few research studies related to the subject. In this regard, it is important that each region has information on the antimicrobial resistance of the area, to guide both empirical and definitive treatment of UTI’s, which is often used indiscriminately.
MATERIAL AND METHODS
Design and study population
The present investigation is an observational cross-sectional analytical type, based on a secondary analysis from a level II-I health institution in the department of Puno, Peru. Which is located in the south-eastern part of the country, which has a population of1 268 441 inhabitants, of which 578 383 are men and 594 314 are women. Regarding age groups, the child population (≤ 9 years old) was 255 604, the young population (10 to 24 years old) 393 743, the adult population (25 to 59 years old) 491 431 and the elderly population (≥ 60 years) of 127 663 17.
We used all records that have been registered in the previously mentioned time period. Of the 3926 records included in the database, 1717 fulfilled our eligibility criteria. We included those patient records that: 1) had a complete urine culture and antibiogram, 2) had positive urine culture results, and 3) had bacteria of the Enterobacteriaceae family as uropathogen. We excluded those records that: 1) did not present complete data on our variables of interest such as age and sex, 2) presented a urine culture with a colony count of less than 10,000 CFU and 3) did not comply in the antibiogram with the total number of antibiotics corresponding to group I of the sensitivity diffusion discs described in the “Manual de procedimientos para la prueba de sensibilidad antimicrobiana por el metodo de disco difusión” of the INS 18.
The outcome variable was defined as antibiotic resistance according to uropathogen. The antibiotics were used according to the procedures described above. Each antibiotic was labeled "S, I and R", explaining the level of sensitivity to the antibiotic where “S” is “sensitive”, “I” is “intermediate”, “R” is “resistant”. These antibiotics were recorded according to uropathogen of the Enterobactericea family (Escherichia coli, Klebsiella Oxytoca and Proteus Vulgaris). The antibiotics were subsequently grouped according to family, which included penicillins (amoxicillin, clavulanic acid, ampicillin/sulbactam and ampicillin), cephalosporins (cefuroxime, cephalothin, cefotaxime and ceftriaxone), quinolones (nalidixic acid, ciprofloxacin and norfloxacin) and others (gentamicin, nitrofurantoin, trimethropim/sulfamethoxazole and amikacin). Finally, for the multivariate analysis, the outcome variable was dichotomized into Resistant and Non-resistant, the first one including the categories of Intermediate and Resistant. In addition, to calculate the intensity of resistance, the new variables mentioned above were used, and resistance was defined as occurring when there was resistance to at least one of the antibiotics of the corresponding family, and sensitive when there was no resistance.
The exposure variables were age and sex. Age is defined as years of life completed and has been categorized into age groups: infant (0 to ≤ 10 years), young (≥11 to ≤24 years), adult (≥25 to ≤59 years) and elderly (≥60 years). And the sex variable was considered as male and female.
Data recollection and analysis
The present study is secondary to a database analysis compiled between the years 2014 and 2017, performed in the institution's laboratory based on the INS "Procedimientos de Laboratorio" protocol. After obtaining the result, the antibiogram was applied to the uropathogen according to the technique of the “Manual de procedimientos para la prueba de sensibilidad antimicrobiana por el metodo de disco difusión” of the INS.
The data was introduced into Microsoft Excel program and quality control was performed; it was exported to the STATA 15 statistical package for statistical analysis. At the descriptive level, the sociodemographic characteristics of the participants were calculated using absolute and relative frequencies for categorical variables such as age group, sex, uropathogen and sensitivity to antibiotics. In relation to the bivariate analysis, the information was used only for Escherichia coli bacteria, using the chi2 test, to analyze the categorical variables of age group and sex, independently, in relation to antibiotic resistance to uropathogens, considering a p < 0.05 as significant. Finally, for the multivariate analysis, a generalized linear model with Poisson family and log link function and robust variances was used to calculate the prevalence ratio (PR) with a 95% confidence interval, where resistance to each family of antibiotics was analyzed by age of uropathogens, adjusted for sex.
Ethical aspects
The present study was approved by the ethics committee of the Universidad Peruana de Ciencias Aplicadas (UPC), Lima and has the authorization of the medical board of the "Las Kalas" Clinic in Puno. The database does not contain personal information of the patients, so their confidentiality and anonymity are guaranteed.
RESULTS
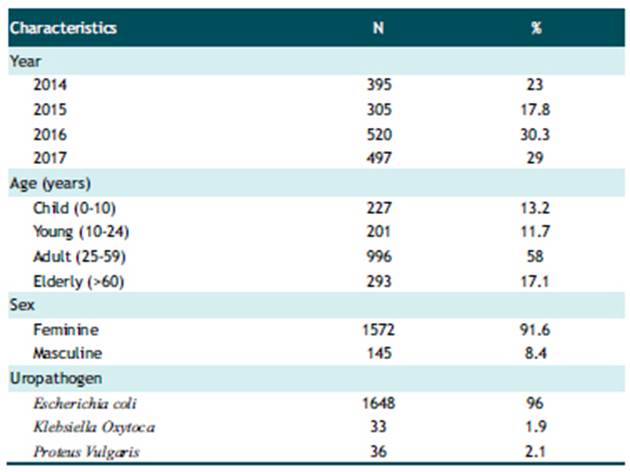
A total of 1717 records of patients with UTI were analyzed. The majority of the population studied was female (91.6%). In terms of age, the majority (58%) belonged to the adult group, followed by the elderly group (17.1%). The most frequent uropathogen was Escherichia coli with 96% of the cases, followed by Proteus Vulgaris with 2.1% (Table 1).
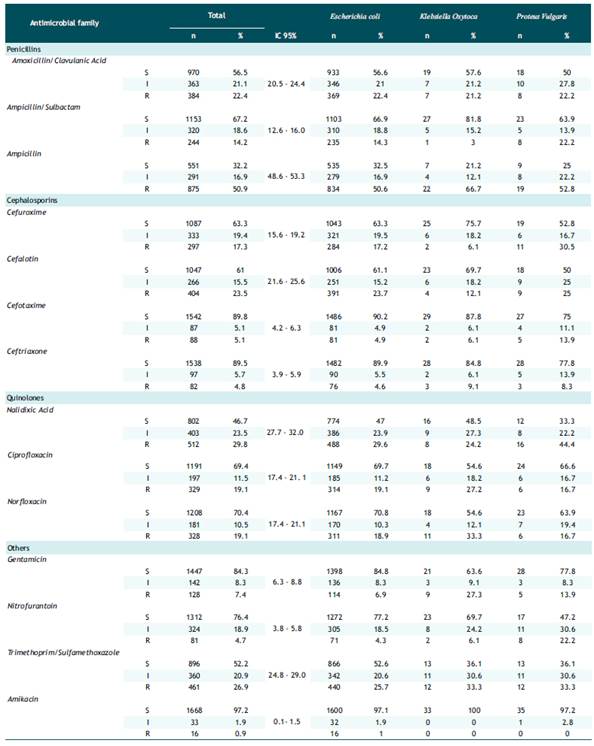
Table 2 showed the total resistance in the penicillin family: amoxicillin/clavulanic acid, ampicillin/sulbactam, ampicillin; presented an overall prevalence of 22.4%, 14.2% and 50.9%, respectively, with a predominance of resistance to ampicillin in Klebsiella oxytoca 66.7%. In the cephalosporin family: cefuroxime, cephalothin, cefotaxime, ceftriaxone; a total prevalence of 17.3%, 23.5%, 5.1% and 4.8%, respectively, was observed. Resistance to cefuroxime in Proteus vulgaris 30.5% was the highest. Likewise, resistance to cefolatine was similar in Escherichia coli (23.7%) and Proteus vulgaris (25.0%). For the quinolone family: nalidixic acid, ciprofloxacin and norfloxacin; an overall prevalence of 29.8%, 19.1% and 19.1%, respectively, was found. Resistance to nalidixic acid was highest in Escherichia coli with 29.6% and Proteus vulgaris with 44.4%. Finally, with the other antibiotics: gentamicin, nitrofurantoin, trimethoprim/sulfamethoxazole and amikacin, the total prevalence was 7.4%, 4.7%, 26.9% and 0.9%, respectively; resistance to trimethoprim/sulfamethoxazole was predominant in Escherichia coli, Proteus vulgaris. Klebsiella oxytoca with 25.7%, 33.3% and 33.3%, correspondingly.
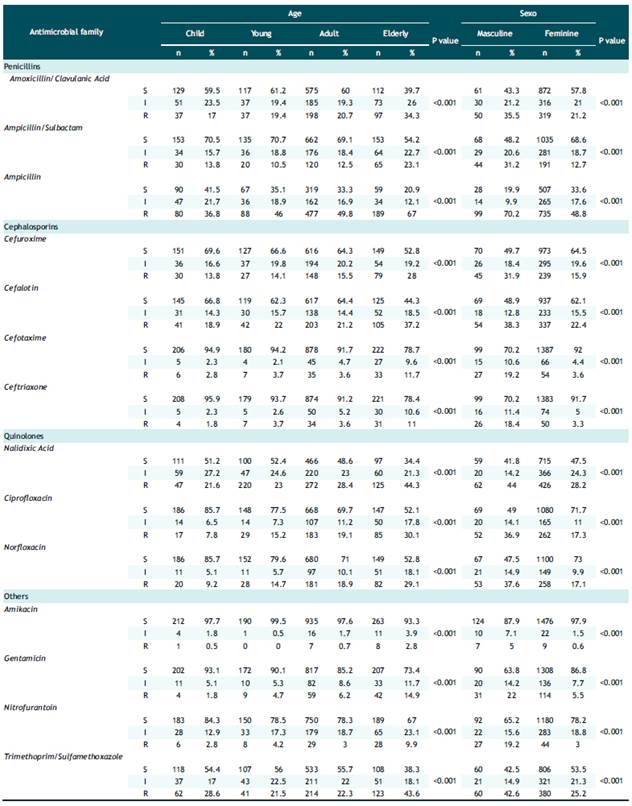
Table 3 presents the antibiotic resistance in Escherichia coli samples and its relationship with age categorized by age groups and sex. All the antibiotics evaluated presented a significant association, both for age (p<0.001) and sex (p<0.001). Likewise, an increase in resistance was observed as age increased with the exception of ampicillin-sulbactam, cephalothin, cefotaxime, ceftriaxone, amikacin, nitrofurantoin and trimethropim/sulfamethoxazole. In addition, in males the prevalence of resistance was higher in all antibiotics, being up to 6 times higher in some drugs, such as: ceftriaxone, amikacin and nitrofurantoin.
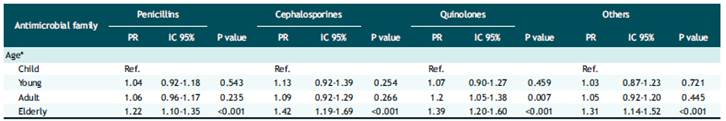
Finally, Table 4 shows the multivariate regression analysis, having as an outcome variable the prevalence of resistance to at least one antibiotic to Escherichia coli in each family. For this analysis, it was decided to classify the variable Resistant/Non-resistant by incorporating the category Intermediate in the Resistant category. The analysis showed that according to age and adjusted for sex, the elderly group was 1.22, 1.42, 1.20 and 1.39 times more likely to be resistant to penicillins, cephalosporins, quinolones, and other antibiotics, respectively, compared to infants.
DISCUSSION
This study found a prevalence of 22.4% resistance to amoxicillin/clavulanic acid in Escherichia coli, very similar to a previous study in Colombia by Castrillon et al. where 28.3% resistance was reported 19. However, this differs from other studies where Abbas et al 20 reported a lower percentage of resistance (11.2%), or the study by Castro-Orozco et al 21, which presented a higher resistance (62.0%). Theoretically, it is established that any antimicrobial that presents a resistance of less than 20% can be used as a therapeutic option empirically, a concept that should be carefully analyzed with this antibiotic 22).
Resistance to third generation cephalosporins such as ceftriaxone was low in Escherichia coli (4.6%) similar to the study by Gómez et al23, who reported 2.8%, which could justify its empirical use in the treatment of urinary tract infections. However, it should be remarked that this study found a high resistance to cefuroxime (17.2%) compared to other studies such as that of Castro-Orozco et al 21 with a resistance of 7.7% and that of Gómez et al23 of 5.6%, which could be controversial and, therefore, opens the need to conduct more studies in Puno and other high Andean regions to make decisions regarding its use.
We found that gentamycin, nitrofurantoin and amikacin have a lower resistance pattern in all of the uropathogens compared to the studies from Abbas et al 20, Castro-Orozco et al 21 and Castrillon et al 19. The high rate of resistance to trimethoprim-sulfamethoxazole by all of the analyzed uropathogens has been widely reported in various investigations that show high bacterial resistance to this antibiotic, despite numerous recommendations on its exclusive use after obtaining an antibiogram that demonstrates its sensibility. For this reason, Puno should reconsider its therapeutic use in UTI.
There is scarce information about the antimicrobial resistance in the peruvian high-andean regions. Escherichia coli had a 19.1% resistance to ciprofloxacin, 26.9% to trimethoprim-sulfamethoxazole and 4.3% to nitrofurantoin, results similar to that reported by Marcos-Carabajal et al 24, in which a 18.4%, 25.5% and 6.1% resistance was respectively observed. Nonetheless, the resistance to ceftriaxone (4.6%) and gentamycin (6.9%) was lower compared to Marcos Carabajl et al 24, who showed an 14.3% and 13.3% resistance respectively.
The present study found that the elderly group had 1.22 times greater probability of being resistant to penicillin, when compared to the infant group, a result similar to that reported by Sanchez et al 25, who found that women older than 65 years had 1.46 times greater probability of presenting resistance to amoxicillin/ clavulanic acid as found by Lee et al 26, where adults over 60 years are 1.07 times more likely to have resistance compared to those under 60. On the other hand, Sotto et al 27, reported that people older than 65 years were 2.19 times more likely to present antimicrobial resistance compared to those below 64. Another antimicrobial family used in the UTI’s treatment are the cephalosporins, our study showed that the elderly group had 1.42 times more probability of presenting resistance to this family when compared to the infant population. Similar to what has been described by Sanchez et al 25, where women of the elderly age group were 1.50 and 2.60 times more likely to present resistance to ceftriaxone and cefuroxime respectively, compared to women below 64.
Finally, our analysis reflected that the elderly age group had 1.39 times more probability to present antimicrobial resistance to other antibiotics compared to the infant population. This result was similar to what has been reported in literature by Lee et al 26, where adults older than 60 years had 1.14 more probability of presenting resistance to amikacin compared to those under 60. Similarly, Treviño et al 28, reported that adults older than 65 years are 1.79 more likely to present resistance to gentamicin compared to those younger than 64 years. On the other hand, Sanchez et al 25 reported that women older than 65 years were 3.14 times more likely to present resistance to nitrofurantoin, compared to those under 64 years of age. The greater antimicrobial resistance in the elderly group may be explained by multiple factors, like a prolonged exposure to antibiotics, which leads to an alteration of their microbiome and vulnerability to a possible later colonization by resistant bacteria 29.
This study has some limitations as the fact of ignoring the clinical context and outcome from the patient, underlying chronic diseases, current status, possible gestation, immunodepression, hospitalization, use of urinary catheter, etc. There is also no clinical diagnosis of the patient. Likewise, the location where the infection was acquired is unknown. Finally, the obtained results can’t be extrapolated to other populations outside from the analyzed area. The bivariate and multivariate analysis of the uropathogens Klebsiella oxytoca and Proteus vulgaris was not performed because the results were not statistically significant. Among the strengths of the study, we must mention that we have a large sample size. Likewise, we found that the robust regression model used has been rarely used in high Andean areas that seek to characterize resistance patterns and identify associated factors. Lastly, the collection urine culture samples allow us to have reliable information about the results, because the international results were met, in turn, specific methods were used for the characterization of sensitivity or resistance, which avoid the possibility of error in the results.
The collected information in this study, in a high Andean area, indicates that the antimicrobial resistance pattern utilized to treat UTI’s, despite being lower than in other areas of the region, is still high and worrisome. Finding that the penicillin family has the highest resistance pattern, therefore it must be used with caution as empirical treatment in this disease.
RECOMMENDATIONS
The results presented in this study allow us to evaluate and demonstrate that the current situation, regarding antibacterial resistance, is similar to the situation at the national or international level, where elderly people present the highest resistance. Research in this area should be improved and applied to other regions of the country in an effort to have more information and be able to create public policies for the appropriate management of antibiotics. In an era where antibiotic resistance is on the rise, the proper use of these drugs is essential to prolong the efficacy of therapeutic agents.