INTRODUCTION
The global spread of antimicrobial resistant microorganisms is currently classified as one of the most important threats to human health 1. These microorganisms include carbapenem-resistant Enterobacteriaceae that are currently present on the list of priority pathogens for research and development of new antibiotics 2,3. Enterobacterales express the OXA-48 enzyme, a class D carbapenemase with hydrolytic activity against carbapenems and indicated as one of the main causes of resistance among Enterobacterales isolates. OXA-48 carbapenemase was initially identified in Turkey in 2001 in a Klebsiella pneumoniae strain 4. Outbreaks involving OXA-48 producing Enterobacteriaceae have been reported in Latin America including Brazil, Argentina, Colombia, Mexico and Ecuador 5. To date, only OXA variants such as blaOXA23, blaOXA24 and blaOXA143 have been reported in Acinetobacter baumannii strains in Peru 6. An outstanding characteristic of this oxacillinase describes that it hydrolyzes imipenem and meropenem with less activity than if it has ertapenem as a substrate 7. We present the first report of 2 systemic infections by OXA-48-producing Klebsiella pneumoniae in Peru. Both cases were admitted in Guillermo Almenara Irigoyen National Hospital, a reference center in social security.
REPORT OF CASE
Case 1
A 31-year-old female patient with a history of kidney-pancreas transplantation due to type 1 diabetes mellitus as a background disease (2018). The patient is presented with chronic dysfunction of the pancreas graft and protein-energy malnutrition (weight 37 kg, height 153 cm, BMI 15.8). She received immunosuppressive treatment with tacrolimus, mycophenolate and prednisone. In addition to what was described, she presented neurogenic bladder, which led to multiple previous hospitalizations for complicated urinary tract infections. Three months before the illness that conditioned the current hospitalization, she was administered meropenem for 26 days because extended-spectrum beta-lactamase (ESBL) producing Klebsiella pneumoniae was isolated twice from urine cultures.
During the course of the disease, she was admitted to emergency with emetic syndrome, fever and abdominal pain lasting one week. Laboratory tests revealed: white blood cell count 10 700/mm3 (0% bands, 27.2% lymphocytes), hemoglobin 11.9 g/dL, platelets 455 000 / mm3, creatinine 0.72 mg/dL, urea 36 mg/dL, albumin 2.2 g/dL, tacrolimus 4.48 ng/L, C-reactive protein 115 mg/dL. Carbapenem-resisting Klebsiella pneumoniae was isolated from blood, central venous catheter and urine cultures; production of Class D OXA-48 carbapenemase was detected. The susceptibility profile is presented in Table 1. Empirical antibiotic treatment was started with meropenem; 3 days later, therapy was redirected by adding colistin for 14 days, with a good clinical evolution and resolution of inflammatory parameters. The clinical discharge was indicated with a urinary catheter, for which she was prescribed prophylactic fosfomycin, 3g weekly for 4 weeks.
Table 1 Microbiological isolation data and results of antimicrobial susceptibility testing (AST).
| Date | Patient 1 | Patient 2 |
| Microorganism | Klebsiella pneumoniae spp pneumoniae | Klebsiella pneumoniae spp pneumoniae |
| Specimen | Urine /Peripheral blood/central venous catheter | Bronchial Fluid |
| Phenotype | Carbapenemase productor OXA-48 + | Carbapenemase productor OXA-48 + |
| Ampicillin / sulbactam | MIC ( 32 (R) | MIC ( 32 (R) |
| Piperacillin/ tazobactam | MIC ( 128 (R) | --- |
| Ceftazidime | MIC = 16 (R) | MIC ( 64 (R) |
| Cefepime | MIC ( 64 (R) | MIC ( 64 (R) |
| Ertapenem | MIC ( 8 (R) | MIC ( 8 (R) |
| Imipenem | MIC ( 16 (R) | MIC ( 16 (R) |
| Meropenem | MIC =8 (R) | MIC ( 16 (R) |
| Amikacin | MIC = 8 (S) | MIC ( 2 (S) |
| Gentamicin | MIC ( 16 (R) | MIC (1 (S) |
| Ciprofloxacin | MIC ( 4 (R) | MIC ( 4 (R) |
| Tigecycline | MIC = 2 (S) | MIC = 4 (I) |
| Colistin* | MIC ( 0.5 | MIC ( 0.5 |
| Trimethoprim / sulfamethoxazole | MIC ( 320 (R) | MIC ( 320 (R) |
Resistance confirmed by VITEK-2.
* Colistin resistance screening by agar-spot.
S = sensitive, I = intermediate, R = resistant.
Case 2
35-year-old female patient with a history of laparoscopic gastric bypass (December 2019) due to grade 2 obesity, and diagnostic laparoscopy and adhesiolysis of postoperative adhesions in the enteroenteric anastomosis (February 2020). She was hospitalized in October 2020 for intestinal obstruction secondary to bowel adhesions and adhesions between the omentum and the abdominal wall, for which she underwent a new laparotomy with intestinal resection and Roux-en-Y reconstruction. Laboratory test results were as follow: white blood cell count 15 700/mm3 (7% bands), hemoglobin 8.2 mg/dL, platelets 262 000/mm3, creatinine 0.5 mg/dL, urea 27 mg/dL, albumin 2.0 g/dL, and C-reactive protein 166 mg/dL. During the post-operative period, the patient had respiratory insufficiency and fever, and received invasive mechanical ventilation. The chest computerized axial tomography showed infiltrates compatible with pneumonia and pleural effusion at the right lung (Figure 1). While in the intensive care unit, carbapenem-resisting Klebsiella pneumoniae with class D OXA-48 carbapenemase was isolated from a bronchial culture; therefore, specific treatment with colistin and high doses of tigecycline were prescribed for 10 days. The susceptibility profile is presented in Table 1. Clinical evolution was favorable, with a decrease in inflammatory parameters and early weaning from mechanical ventilation. The patient continued with parenteral nutrition and outpatient surgical follow-up.
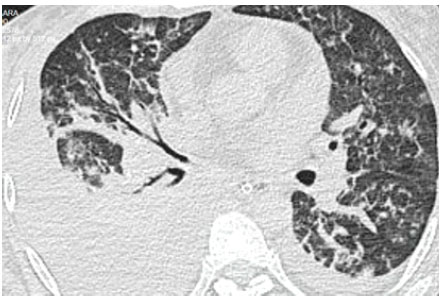
Figure 1 Case 2. Chest computerized axial tomography showing infiltrates compatible with pneumonia and pleural effusion at the right lung
In both cases, the initial identification of the germ and the antimicrobial susceptibility test (AST) were carried out using VITEK2. The presence of OXA-type carbapenemases was detected using the modified carbapenems inactivation method [mCIM], disc synergy test using boronic acid and rapid immunochromatographic test (K-set CORIS Bio-Concept RESIST-4 O.K.N.V). Molecular confirmation was carried out by conventional PCR for the detection of blaOXA-48 resistance genes. It was not possible to perform genomic sequencing due to the lack of appropriate equipment and limited resources during the COVID-19 pandemic.
During hospitalization, contact precautions and infection control measures were maintained to prevent secondary cases.
Ethical approval
This case report has not required approval by the ethics committee from the hospital where it was performed. This is because the case was described as part of the hospital epidemiological surveillance program for multi-resistant microorganisms. The patients signed an informed consent for this work.
DISCUSSION
To our knowledge, this is the first report of systemic infections caused by OXA-48-producing Klebsiella pneumoniae in Peru. The presence of OXA-48 carbapenemase in Klebsiella pneumoniae Sequence Type 307 has recently been described in an immunocompromised patient from Ecuador with previous hospitalization in Ukraine, which suggests a probable mechanism of imported resistance 8. Our patients reported no recent travel history; however despite hospital and community isolations, we do not rule out other sources of infection. OXA-48 has previously been detected in feces from swine, wild boar and sewage treatment plants. Similar sources of infection have not yet been investigated in Peru 9.
RESIST-4 O.K.N.V is a lateral flow immunochromatographic assay for the rapid detection of OXA-48. Similar results were obtained by this test and PCR in both samples. Although MacDonald et al. reported a 100% predictive value for OXA-48 using this test 10, other studies reported false negatives when double carbapenemases (eg NDM / OXA) were produced. Xpert Carba-R would be a good option to validate locally. 11
We consider important the implementation of rapid molecular tests in local laboratories. However, whereas the prevalence of CPE infections in Peru remains uknown, the routine use of these tests may not be cost effective. These tests may be used based on adequate clinical judgment, 12 as the cases presented in this study.
Recommended treatment options for OXA-48 infections are ceftazidime / avibactam (CAZ /AVI), cefedirocol, tigecycline, eravacycline 13. However, other options such as carbapenems, colistin, fosfomycin and aminoglycosides are used in monotherapy or in combination with heterogeneous survival rates with a limited number of patients included. (14) Sousa et al. reported in a cohort study that included 57 patients with systemic OXA-48 infections who received CAZ / AVI as rescue, clinical, microbiological cure and mortality at 30 days in 80%, 67% and 22% respectively, without differences between combination therapy or monotherapy. 15 In both isolates ESBL was detected, which limits the options of ceftazidime and cefepime in OXA-48, usually not hydrolyzed. 14
To date, Peru does not have other therapeutic options such as parenteral fosfomycin and CAZ/AVI implemented in public hospitals. A combined colistin strategy was used for treating both patients. Its limitations are the lack of a susceptible category according to the Clinical & Laboratory Standards Institute (CLSI) standards 16, renal toxicity and a mortality even higher than 50% if the focus of bacteremia is urinary when combined therapy with carbapenems is not included 17. In case 2 the treatment included high doses of tigecycline showing a good clinical response. The in- vitro susceptibility of tigecycline varies in the literature with MIC ranges between 0.12-8 and a moderate success in double or triple therapy 14.
Risk factors for colonization by OXA-48 producing Enterobacteriaceae includes: ICU stay for more than 72 hours, mechanical ventilation, previous treatment with carbapenems or aminoglycosides, use of antacids, prolonged hospital stays 7. On kidney transplant patients, infections caused by carbapenemase-producing Klebsiella pneumoniae may occur in early transplantation up to 185 days later 18. A selection pressure due to the prolonged hospitalization and use of carbapenem may have been a possible risk factor in case 1. Due to the risk of cross infection, the implementation of CPE colonization screening in organ donors should be considered 19.
Infections by CPE in surgical patients are associated with higher mortality, longer hospital stay and higher costs. Mora Guzmán et al. reported that more than 70% of patients with intraabdominal infections by OXA-48-producing Enterobacteriaceae had taken carbapenems in the last 30 days. Besides, risk factors associated with mortality were septic shock, blood transfusion, immunodeficiency, solid tumor and metastatic disease 20. It has not yet been determined whether a history of multiple surgeries could be a factor for colonization with OXA-48. However, this may have contributed to a longer stay in case 2.
CONCLUSIONS
Overall, the identification of OXA-48-producing Klebsiella pneumoniae in Peru highlight the need of optimized molecular diagnostic tools for the detection of carbapenemases. These findings evidence the importance of establishing a national antimicrobial resistance surveillance network integrated with prevention and control programs to avoid a possible transmission of resistance genes between species















