Introduction
According to the World Report on tuberculosis of the World Health Organization (WHO) 2022 1, 25% of the world population has immunological evidence of contact and previous infection by mycobacterium tuberculosis and in 2020, 10 million people developed the active form of the disease 2. Multidrug-resistant tuberculosis (MDR-TB), defined as Mycobacterium tuberculosis resistant to both isoniazid and rifampicin, and extensively drug-resistant tuberculosis (XDR-TB) defined as M. tuberculosis resistant to isoniazid, rifampicin, second-line injectables and a fluoroquinolone) are a profoundly severe global public health problem with both often being treated with second-line injectables (SLIs), aminoglycoside: amikacin, streptomycin, kanamycin, and the polypeptide capreomycin 3,4. WHO currently estimates that the global proportion of rifampicin-resistant TB is 3% among new TB cases and 18% among previously treated TB cases, resulting in nearly half a million cases per year. The three countries with the highest proportion of MDR-TB are India, China, and the Russian Federation 5.
In 2018, the World Health Organization (WHO) proposed replacing SLIs with oral alternatives (e.g., bedaquiline, delamanid). However, the affordable standardized treatment regimens continue to use amikacin and streptomycin for at least 6 to 8 or even more months6. Their use is sometimes limited due to serious adverse events such as ototoxicity and hearing loss7. Globally, there is wide variation in the incidence of ototoxicity and hearing loss in patients with MDR/XDR-TB secondary to SLIs, which are among the devastating consequences of these diseases affecting between 50 to more than 60% of patients treated with SLIs8. In addition, cochlear implants may be indicated in patients with severe, unilateral/bilateral hearing loss with no benefit with conventional hearing aids, but its use in tuberculosis patients is uncommon 9.
We report the first case of right unilateral cochlear implantation performed in a Peruvian 34-year-old female who had never used hearing aids before the cochlear implant, a patient in whom XDR-TB was resolved aminoglycoside-induced severe and permanently 24-moth of history disabling ototoxicity developed. The patient provided informed consent.
Case report
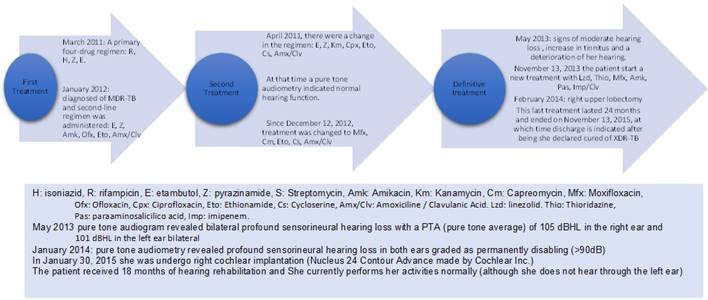
In March 2011, she was diagnosed with only Acid-Fast Bacilli (AFB) smear-positive pulmonary tuberculosis involving her right lung and pleura. After evaluation of renal function through serum concentrations of urea and creatinine, which were normal, a primary four-drug 6-month regimen was started consisting of rifampicin, isoniazid, pyrazinamide, and ethambutol (no drug susceptibility testing was performed because this was the first episode). In January 2012, she was diagnosed with AFB smear and culture MDR-pulmonary TB. Second-line treatment was administered based on culture, and antimicrobial sensitivity results in sputum and ethambutol, pyrazinamide, amikacin, ofloxacin, ethionamide and amoxicillin/clavulanate were administered. Unfortunately, in Peru tests are not available to assess drug concentrations in clinical situations, so the patient was not subjected to such procedures 10. In April 2012, the treatment was changed to ethambutol, pyrazinamide, kanamycin, ciprofloxacin, ethionamide, cycloserine and amoxicillin/clavulanate because, in the upper zone of the right lung, active infiltrates. A cavitary lesion had also developed (the standardized treatment in Peru for these cases). In 2012, meropenem or imipenem was not used in Peru, and amoxicillin/clavulanate was administered alone). At that time, conventional pure tone audiometry indicated "normal hearing function", and no signs or symptoms of sensorineural hearing dysfunction were reported. In December 2012, the treatment was changed again to moxifloxacin, capreomycin, ethionamide, cycloserine, and amoxicillin/clavulanate based on the results of an antimicrobial sensitivity test performed in a sputum sample from July 2012. This test showed resistance to isoniazid, rifampicin, ethambutol, pyrazinamide, streptomycin with antimicrobial sensitivity to ciprofloxacin, kanamycin, and amikacin. Unfortunately, this culture had to be done immediately after the sputum sample was obtained in July 2012. This could have contributed to the patient becoming a case of XDR-TB.
In March 2013, the result of another antimicrobial sensitivity test of a sputum sample from December 2012 showed resistance to isoniazid, rifampicin, ethambutol, streptomycin, amikacin, capreomycin with susceptibility to pyrazinamide, ciprofloxacin, and kanamycin, indicating the presence of pre-XDR-TB6. In a computed tomography scan of the lungs performed in April 2013, bilateral lung lesions indicated disease progression. In May 2013, the case was classified as a failure after two treatments. Throughout this period, monthly sputum cultures were positive. In May 2013, during the usual conversation, signs of moderate hearing loss were detected. The patient reported an increase in tinnitus and a deterioration of her hearing (In those years, regular monitoring of auditory function was not frequent). In September 2013, a new antimicrobial sensitivity test was received from a sputum culture March 2013. The results showed resistance to isoniazid, rifampicin, ethambutol, pyrazinamide, streptomycin, kanamycin, amikacin, capreomycin, cycloserine, and ciprofloxacin, indicating that the patient was now a carrier of XDR-TB (between December 2012 and October 2013, the patient continued receiving moxifloxacin, capreomycin, ethionamide, cycloserine, and amoxicillin/clavulanate). The decision was made to hospitalize the patient and initiate in November 2013 a new treatment with linezolid, thioridazine, moxifloxacin, amikacin, and imipenem/clavulanate (despite the result of the sensitivity test, the use of amikacin was continued). The monthly sputum culture results were negative from the second month of treatment. In February 2014, a right upper lobectomy was performed (which probably played a fundamental role in the success of the treatment). This last treatment was maintained for 24 months and ended in November 2015, at which time the patient was discharged and declared cured of XDR-TB 1 (Figure 1).
In April 2012, the first conventional pure-tone audiometry indicated "normal hearing function", and no signs or symptoms of sensorineural hearing dysfunction were reported. Her second pure-tone audiogram report in May 2013 revealed bilateral profound sensorineural hearing loss with a pure-tone average of 105 dBHL in the right ear and 101 dBHL in the left ear. In January 2014, pure-tone audiometry revealed a profound sensorineural hearing loss in both ears graded as permanently disabling (> 90dB). At that time, the patient only used hand signs and writing for communication. A Cochlear implant is the only possible therapeutic intervention for this patient with severe-profound bilateral sensorineural hearing loss of cochlear origin. This procedure had not been performed before in Peruvian patients who had been treated for tuberculosis 11-13.
In January 2015, the patient underwent right cochlear implantation (Nucleus 24, Contour Advance made by Cochlear Inc.). A standard postauricular transmastoidectomy facial recess approach was used to insert the device. After the cochlear implant placement, the patient received 18 months of hearing rehabilitation. Currently, she performs her activities normally and without limitations (although with no hearing in the left ear). She has finished her studies in physiotherapy and rehabilitation, intending to help other people affected by aminoglycoside-induced diseases (Figure 2). We do not have information about perception speech score before and after cochlear implant, either in the closed or open set, but clinically she feels great
Discussion
Tuberculosis and its treatments continue to be challenging and complex, especially in developing countries, where the disease is still highly prevalent 14. It is even more complex during 2020-2021 due to the interaction with COVID-19 15 and its impact on specific populations 16,17. This disease is among the most critical threats to public health also in Peru 18. In addition, serious adverse effects, such as severe ototoxicity, are complex challenges.
Between 1999 and 2009, 110 patients received a cochlear implant in Peru, and between 2006 and 2015, 108 cochlear implants were performed, only 12 of which were in adults. Adults with severe hearing loss are not usually considered candidates for this treatment10. This procedure is even less frequent in adults with severe-profound bilateral sensorineural hearing loss of cochlear origin secondary to aminoglycosides as a component of treatment for pulmonary tuberculosis12.
The following mechanisms contribute to aminoglycoside-induced toxicity after systemic administration: 1) drug transfer across endothelial and epithelial barrier layers; 2 sensory cell uptake of these drugs; and 3) disruption of intracellular physiological pathways related to the cumulative dose of the drug and the time of use6. Therefore, aminoglycoside-induced ototoxicity can only be avoided by not taking these drugs to treat drug-resistant tuberculosis 19, which was not possible in the present case because new drugs such as bedaquiline and delamanid were not yet available for the treatment of MDR /XDR-TB7,8.
Since 2018, the WHO no longer recommends kanamycin and capreomycin. In addition, streptomycin, and amikacin, which belong to group C drugs for the treatment of MDR/XDR-TB, should only be used when a treatment scheme with 4 to 5 group A and B drugs is not possible 6. The use of amikacin and streptomycin is now only recommended if the bacteria are still sensitive to these drugs and if the hearing function can be monitored monthly throughout their time of use3. Some authors also recommend that these drugs only be used if serum concentrations can be monitored to avoid toxic concentrations 10.
This cochlear implant operation is an important landmark in managing tuberculosis treatment sequelae in Perú and Latin America11. However, while this is "the proverbial drop in the ocean" concerning the need for otologic care in many tuberculosis patients with treatment-induced ototoxicity, it represents an essential first step towards meeting this critical need 12.
Systematic monitoring of AEs during and after treatment needs to be strengthened in most TB programs. It is essential to monitor hearing loss and kidney function. In the event that hearing loss has occurred, it is necessary to carry out all possible procedures to recover this essential function of people affected with tuberculosis.