Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.62 no.4 Lima oct. 2016
SIMPOSIO EMERGENCIAS Y COMPLICACIONES SEVERAS OBSTÉTRICAS
The morbidly adherent placenta
La placenta mórbidamente adherida
Tripp Nelson, MD1
1 Division of Maternal-Fetal Medicine, Medical University of South Carolina, USA
Abstract
The incidence of morbidly adherent placentation has increased in the current era of obstetrics paralleling the cesarean rate. The problem of abnormal placental adherence is a significant contributor to maternal morbidity and requires multi-disciplinary care for management. The epidemiology, antenatal diagnosis, and a multidisciplinary care model are presented in this review. Multiple methods of imaging are reviewed in detail. In addition, both surgical and non-surgical interventions for management of the abnormally adherent placenta are evaluated.
Keywords: Placenta Accreta; Placenta Increta; Placenta Percreta; Cesarean Hysterectomy; Obstetric Hemorrhage
Resumen
La incidencia de placentación adherida mórbidamente ha aumentado en la era actual de la obstetricia, en paralelo con la tasa de cesárea. La adherencia placentaria anormal contribuye significativamente a la morbilidad materna y requiere un manejo multidisciplinario. En esta revisión se presenta la epidemiología, diagnóstico prenatal y un modelo de atención multidisciplinario. Se revisa en detalle los varios métodos de imágenes utilizados en el diagnóstico. Y se evalúa las intervenciones quirúrgicas y no quirúrgicas para el manejo de la placenta adherida anormalmente.
Palabras clave: Placenta Accreta: Placenta Increta; Placenta Percreta; Cesárea Histerectomía; Hemorragia Obstétrica.
Introduction
The morbidly adherent placenta is meant as a broad term to signify abnormal attachment and invasion by the placenta to varying degrees (accreta, increta, and percreta). The incidence of morbidly adherent placenta has been steadily increasing in parallel to the increasing cesarean rate worldwide. A recent World Health Organization study reviewed the cesarean rates in 137 countries, finding that 69 had rates above 15%, with many countries exceeding 30-35%(1). In the past 50 years, there has been a 10 fold increase in the incidence of placenta accreta due to an increase in cesarean delivery incidence and advancing maternal age, with a range from as low as 1 per 2 500 to as high as 1 per 530 deliveries(2,3). The significance of this increase cannot be under estimated given the associated drastic increase in maternal morbidity (massive hemorrhage, need for large volume blood transfusion, intra-abdominal infection, ureteral and bladder damage, prolonged need for mechanical ventilation, ICU admission, and fistula formation) and a mortality rate as high as 7%(4). In order to combat this phenomenon, significant efforts have been made to improve antenatal diagnostic capabilities, pregnancy management, operative strategies, and postoperative management. However, perhaps the most important consideration is in the ongoing campaign to prevent the initial cesarean section(5).
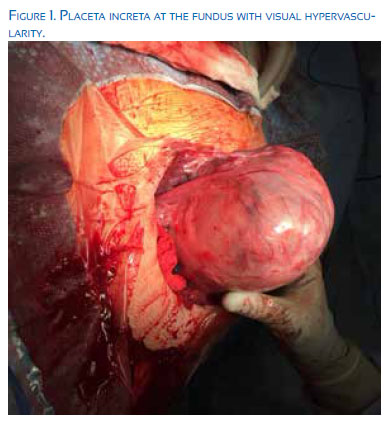
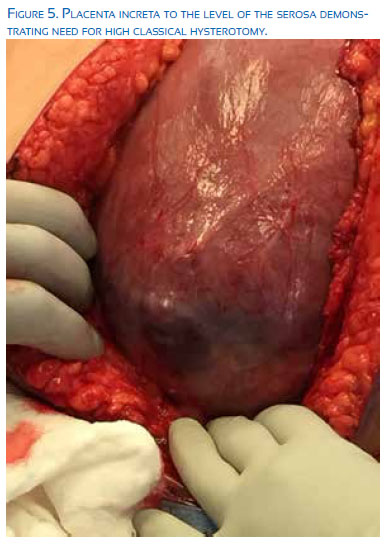
Typically, the maternal morbidity and mortality will increase exponentially with the depth of abnormal placental invasion through the decidua basalis of fibrinous Nitabuch layer. Types of abnormal placental invasion include: 1) placenta accreta –a partial, but not full thickness invasion into the myometrial layer, occurring in 82%, 2) placenta increta – full thickness myometrial invasion without penetration through the serosa layer, representing 12%, and 3) placenta percreta – placental invasion through the uterine serosa or into adjacent pelvic structures, comprising the final 6%.
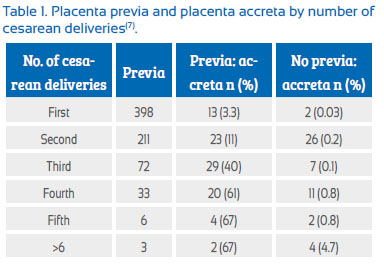
The known risk factors for morbid placental adherence include prior cesarean section, prior uterine curettage or prior uterine surgery for other indications (such as myomectomy), prior endometrial ablation, uterine embolization, and prior pelvic irradiation. The greatest risk factor is the combination of known prior uterine incision in the presence of a placenta previa(6). A 2006 cohort study has estimated the risks of placenta accreta in populations with and without a concomitant placenta previa:
The majority of morbidly adherent placentation occurs in multiparous patients with at least one prior cesarean section. Other risk factors identified in some, but not all studies include advanced maternal age and increasing parity(8,9).
Regardless of pathophysiology (which remains unclear) and risk factors leading to the development of the morbidly adherent placenta, the marked increase in maternal morbidity and mortality demands careful consideration in diagnosis, pregnancy management, and ultimate delivery planning in an attempts to mitigate the many increased maternal and fetal risks associated with this condition.
Diagnostic Imaging
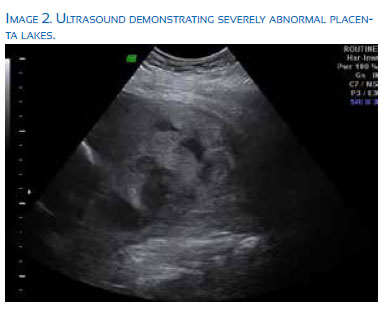
Traditionally, the likelihood of abnormal placental invasion has been based on the clinical history of the patient (number of prior cesarean sections) and the location of the placenta on the uterine wall. Patients with placenta previa and prior cesarean sections have been shown to have an increased risk of placenta accreta based on retrospective data analysis(10). Ultrasound has also proved extremely helpful in further qualifying the risk for placenta accreta based on the presence or absence of certain characteristics, such as the presence of placental lacunae, abnormal placental vascularity as seen on color Doppler, loss of the retro placental clear zone, and myometrial thinning. Multiple prior studies have evaluated the sensitivity and specificity of ultrasound for accreta diagnosis and several recent studies demonstrated sensitivity of 70-80% and specificity as high as 90+%(11-13). Generally, these numbers depend greatly on the location of the placenta with posterior placental location demonstrating increased difficulty in accurate diagnosis, as well as on the overall quality of imaging and experience of the specialist interpreting this imaging.
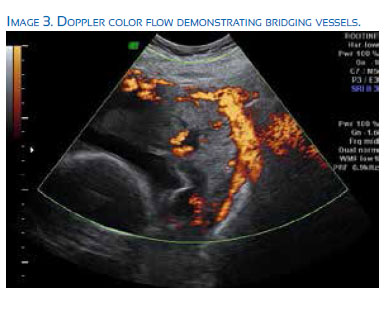
Recently, Martha et al. have published the development of the placenta accreta index (PAI), a mathematical calculation utilizing history of previous cesarean section and placental location along with 4 highly correlated ultrasound indicators to improve the antenatal accuracy of accreta diagnosis(14). Ultrasound factors were developed using linear regression modeling, multi-parametric analysis, and ROC modeling to improve prediction of abnormal placental invasion. The individual parameters that make up the PAI are number of previous cesarean deliveries, presence of grade 2 or 3 lacunae, myometrial thickness, placental location, and presence of bridging vessels. After completing a retrospective analysis of 184 pregnancies with >1 previous cesarean delivery and presence of a placenta previa/low lying placenta, sensitivity, specificity, and probability of invasion were calculated for each PAI score. A score of <3 demonstrated at 19% probability of invasion with a sensitivity of 93%, where as a score >8 has a 96% probability of invasion with a specificity of 100%(14).
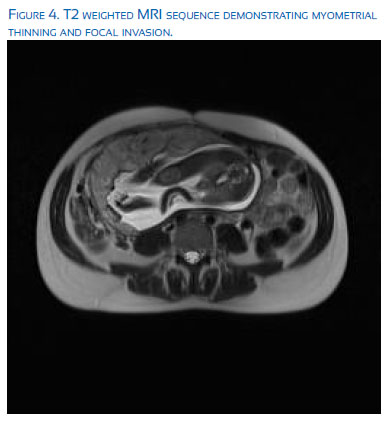
Magnetic resonance imaging (MRI) has also been proposed as an imaging modality that could improve the certainty of diagnosis of abnormal placental invasion. Unfortunately, MRI imaging of the pelvis is limited by the gestational age at the time of imaging, the placental location, maternal BMI, and physician/radiologist expertise in interpreting the imaging, and thus has not been shown to be superior to US as the gold standard for diagnosis. It is an important and often helpful, adjuvant imaging tool, when available. The most important criteria for the diagnosis of a morbidly adherent placenta include: T2 dark intra-placental bands, heterogeneous placenta, abnormal uterine bulging, adjacent organ invasion, and focal interruption of the myometrial wall(15). Ultrasound is typically preformed at 18-20 weeks for the assessment of fetal anatomy and placentation; however, at more advanced gestations or in the setting of equivocal ultrasound findings, MRI can be an excellent complimentary tool. There have been over 11 studies evaluating the use of MRI for the detection of invasive placentation, most of which are retrospective in nature. In general, the sensitivity of MRI has been estimated to be 78-93% with specificity of 77-100%. MRI is extremely use-ful in the evaluation of posterior placentation, when ultrasound imaging is particularly difficult and less accurate(16). Imaging with MRI is typically carried out at 28-32 weeks gestation with considerations for supine maternal positioning in the left lateral decubitus position and a feet first approach to maximize maternal comfort and minimize maternal distress. The bladder should be partially full to optimize imaging of the uterine bladder interface. Routinely, T2-weighted sequences are obtained in the coronal, axial, and sagittal planes. Additionally, a fat saturated T1-weighted in phase gradient sequence can be used to evaluate hyperintense hemorrhage(17).
Accurate antenatal diagnosis of placenta accreta is of paramount importance for multi-disciplinary planning leading up to delivery, patient transfer to tertiary care centers capable of managing massive blood loss, and decision making regarding timing of delivery. Sever al studies confirmed decreased hemorrhage and other maternal complications in cases diagnosed antepartum rather than intrapartum(18-21). Appropriate exclusion of this diagnosis is equally important in order to prevent un-necessary iatrogenic prematurity, invasive maternal procedures in anticipation of blood loss, and inappropriate allocation of hospital resources.
Pregnancy management
There has been little agreement or standardization of the management of antenatally diagnosed morbidly adherent placenta. However, there has been significant discussion in the recent literature to move care of such patients to centers with significant experience in the management of abnormally adherent placentation. Silver et al have put forth the following suggested criteria for accreta centers of excellence(22):
1. Multidisciplinary team
- Experienced Maternal-Fetal Medicine obstetrician
- Imaging expert (ultrasound)c.
- Pelvic surgeon (Gynecologic Oncology or urogynecology)
- Anesthesiologist (Obstetric or Cardiac Anesthesia)
- Urologist
- Trauma or general surgeong.
- Interventional radiologisth.
- Neonatology
2. Intensive care unit and facilities
- a.Interventional radiology
- b.Surgical or medical intensive care unit
- a. 24 hour availability of intensive care specialists
- c. Neonatal intensive care unit
- a. Gestational age appropriate for neonate
3. Blood services
- a. Massive transfusion capability
- b. Cell saver and perfusionist
- c. Experience and access to alternate blood products
- d. Guidance of transfusion medicine specialists or blood bank pathologists
A study at the University of Utah involving 141 accreta cases managed in this multidisciplinary fashion demonstrated a decreased likelihood of requirement of large volume blood transfusions, need for a second surgical procedure, and lower composite morbidity than management with standard obstetric care(23).
In addition to the goal of antenatal diagnosis, the goal of antenatal care should be to balance the risks of prematurity while avoiding the need for emergency delivery due to labor or bleeding. In general, patients may be managed in the outpatient setting without evidence of bleeding or preterm labor. In order to risk stratify patients early in the pregnancy, transvaginal ultrasound can be utilized to evaluate the cervical length at 24-28 weeks gestation. A third trimester cervical length less than 30mm is associated with a higher risk of hemorrhage, uterine activity, and preterm birth(24). Patients with a cervical length of less than 30mm warrant closer observation and a low threshold for inpatient admission. In the absence of any symptoms of bleeding or preterm labor, patients are generally scheduled for cesarean delivery at 34-35 weeks(25) to avoid any increased risks of labor or bleeding beyond this gestational age. Amniocentesis is not preformed prior to this indicated preterm delivery. Rather, patients are given a course of betamethasone approximately 48 hours prior to the scheduled surgery date for the purposes of aiding pulmonary maturity. This practice has been supported by a recent study demonstrat ing the efficacy of late preterm steroid use in the reduction of severe respiratory complications, TTN, surfactant use, and broncho-pulmonary dysplasia(26).
Surgical management
The optimal surgical approach to placenta accreta continues to be debated and remains controversial. Ideally, the delivery of a suspected accreta should occur in a tertiary center with the capabilities described above in a ‘Center of Excellence’. For certain, the situation necessitates large bore IV access, the ability for rapid transfusion, and availability of significant blood products. Anesthesia consultation should be sought pre-operatively whenever possible; however, the potential for large blood loss and hemodynamic instability will often necessitate the use of general endotracheal anesthesia. Most expert recommendations for surgical approach suggest a skin incision which will allow adequate visualization, either midline vertical incision or low transverse incision with the ability to modify exposure with a Cherney or Maylard incisions. The specific placental location should be mapped with ultrasound either prior to surgery or intra-operatively, with the hysterotomy placement to avoid disruption of the placental bed. In many cases this may require a trans-fundal or classical hysterotomy.
Many surgeons have advocated the use of ureteral stent placement pre or peri-operatively to reduce the risk of ureteral injury, especially in the setting of suspected percreta, which can significantly distort the lower pelvic vasculature. A 2009 retrospective study by Eller et al. demonstrated a non-significant reduction in ureteral injury of 0 vs. 7% (p=0.31) when stents were placed prior to skin incision(27). Despite a lack of clear evidence demonstrating improved outcomes with ureteral stenting, a recent survey of obstetricians demonstrated 26% utilized adjuvant ureteral stents in the management of placenta accreta(28). When considering ureteral stent placement the depth of placenta invasion and pelvic extension should be weighed with the risks of additional anesthesia time and complications of stent placement.
Another adjuvant procedure considered peri-operatively is the use of balloon occlusion of either the uterine arteries or internal iliac arteries for the purpose of decreased perfusion of the uterus. In general the balloons are placed but not inflated prior to scheduled surgery in an interventional radiology suite, and inflated at the time of hysterectomy. However, with the advances of combined IR operative suites, some centers have the added advantage of balloon placement in the same room as the planned delivery. Again, the risks and benefits of vessel occlusion techniques is a hotly debated topic. The risks of these procedures include arterial damage, infection, and inadvertent vessel occlusion. Additionally, critics have always cited the significant collateral blood flow to the uterus and lack of efficacy of hypogastric artery ligation as significant limitations of this approach. A small prospective observational study demonstrated a reduction in both mean estimated blood loss (933 mL vs. 1 507 mL) and transfused blood products (0.67 units vs. 3.31 units) in a subset of patients with placenta percreta treated with pre-operative balloon catheter placement of the internal iliac arteries versus standard multi-disciplinary cesarean hysterectomy alone(29). However, multiple case reports exist also describing significant complications and decreased utility with the use of this technique, including one description of bilateral pseudo aneurysms, unilateral arterial rupture, and thrombus formation compromising the lower extremity in a patient who suffered massive hemorrhage despite the intervention(30). Given the current paucity of information regarding these techniques, patient specific characteristics (such as pre-operative hemoglobin and willingness to accept transfusion), placental imaging, and interventional radiology experience with such techniques at the local institution should be used to individualize care.
Conservative management
The conservative management of placenta accreta can be viewed in two separate lights: conservation for the purposes of desired continued fertility and conservative in situations of undiagnosed accreta (without proper resources in place) or extreme percreta deemed unresectable. In cases of focal or limited area of abnormal placentation, uterine conservation has been quite successful with the use of curettage of the affected area after placental delivery or the use of a wedge resection technique with repair similar to that undertaken during a myomectomy with multi-layer closure of the remaining myometrium and serosa. However, in cases of more deeply or widely invasive placentation these techniques are not feasible.
In cases of significant accreta where future fertility is desired, a fundal hysterotomy is preformed, the cord is then ligated and returned to the uterus, and finally the hysterotomy is closed with the goal of allowing placental reabsorption to occur over time. A retrospective multicenter French trial of 131 women demonstrated relative success with 75% of patients having total placental reabsorption at an average of 13.5 weeks (range 4-60 weeks)(31). However, severe maternal morbidity was noted in 6% of cases, including 7 cases of sepsis, 3 cases of venous thromboembolism /pulmonary embolism, and 1 maternal death. Other studies commonly list delayed hemorrhage, DIC, endomyometritis, and sepsis as the major potential complications. Of note, in this particular study only 28% of patients had more than 1 prior cesarean delivery. When counseling patients on the risks and benefits of conservative management for fertility, consideration must also be given to the recurrence risk of abnormally adherent placentation in future pregnancies. A recent meta-analysis demonstrated a pooled recurrence risk of at least 20%(32).
In cases of conservative management due to unsuspected diagnosis of accreta or percreta deemed unresectable at the time of initial surgery, there are many adjuvant therapies that have been employed. These include the use of uterotonic medication, prophylactic antibiotic therapy, prophylactic uterine artery embolization, and methotrexate administration. There is no uniform consensus on a ‘best practice’ regimen nor is there strong literature support for or against the vast majority. The exception is the use of methotrexate therapy to aid in placental absorption. Historically, this practice has been in physiologic doubt due to the low level of trophoblastic division in the third trimester. A further contributing factor to the use of methotrexate is its contra-indication with breast-feeding which is known to improve long term neonatal and infant outcomes. There are some mixed results of the use of methotrexate in the literature, but the largest cohort of conservatively managed patients demonstrated "no convincing evidence currently supports the efficacy of methotrexate in cases of placenta accreta left in situ…"(33). Additionally, the potential adverse effects of methotrexate administration include the potential for nephrotoxicity and bone marrow suppression leading to pancytopenia.
A final consideration in the conservative management of placenta accreta, despite the number of adjuvant therapy or protocol utilized is the potential for sudden, catastrophic bleeding requiring emergent reoperation and hysterectomy. A review by Pather of 57 cases of conservatively managed placenta percreta demonstrated that 60% required further surgery, 40% of which were emergent hysterectomy(34). Another review by Clausen et al. of 119 cases demonstrated a similar risk of delayed hysterectomy (58%) which were completed in an emergent fashion 85% of the time(35). Due to these risks, some have advocated for planned delayed hysterectomy, not truly a conservative measure, in order to attempt to balance the potential of hemorrhage risk at initial hysterectomy with the longer term risks of sudden hemorrhage requiring emergent or unplanned reoperation. There is little available data on this hybrid approach or the optimal time for interval hysterectomy. A combination of recurrent imaging with ultrasound MRI as well as the trending of beta-hCG levels has been employed in the past to help guide the timing of interval hysterectomy (aiming for a balance of placental reabsorption and resolution of pelvic hyper-vascularity) but this is not a widely employed strategy and does not obviate the risk of ongoing complications in the intervening time.
Summary
The appropriate diagnosis and management of the morbidly adherent placenta remains a conundrum for the obstetrician despite advances in care. The scope of the problem will continue to increase along with the cesarean delivery rate. What is clear is that a high clinical index of suspicion in patients with risk factors and careful evaluation of imaging is vital to the antenatal diagnosis and will subsequently improve patient outcomes. In addition, timely recognition will allow referral to maternal-fetal medicine subspecialists capable of coordination of complex multi-disciplinary care in tertiary centers capable of dealing with patients in a critical care setting both pre and post-operatively.
References
1. Gibbons L, Belizan JM, Lauer JA, Betran AP, Merialdi M, Althabe F. The global numbers and costs of additionally needed and unnecessary cesarean sections performed per year: Overuse as a barrier to universal coverage. World Health Report (2010) Background Paper, No 30. [ Links ]
2. Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty year analysis. Am J Obstet Gynecol. 2005;192:1458- 61. DOI:10.1016/j.ajog.2004.12.074. [ Links ]
3. Miller DA, Chollet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177:210-4. [ Links ]
4. O’Brien JM, Barton JR, Donaldson ES. The management of placenta percreta: conservative and operative strategies. Am J Obstet Gynecol. 1996;175:1632-8.
5. Spong CY, Berghella V, Wenstrom KD, Mercer BM, Saade GR. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol. 2012 Nov;120:1181-93. DOI:10.1097.AOG.0b013e3182704880. [ Links ]
6. Silver RM, Barbour KD. Placenta accreta spectrum: accreta, increta, and percreta. Obstet Gynecol Clin North Am. 2015 Jun;42(2):381-402. DOI: 10.1016/j.ogc.2015.01.014. [ Links ]
7. Silver RM, Landon MB, Rouse DJ, et al; National Institute of Child Health and Human Development Maternal- Fetal Medicine Units Network. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107(6);1230. DOI:10.1097/01. AOG.0000219750.79480.84. [ Links ]
8. Bowman ZS, Eller AG, Bardsley TR, Greene T, Varner MW, Silver RM. Risk factors for placenta accreta: a large prospective cohort. Am J Perinatol. 2014;31(9):799-804. DOI:10.1055/s-0033-1361833. [ Links ]
9. Miller DA, Chollet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997; 177(1): 210-4. [ Links ]
10. Silver RM, Landon MB, Rouse DJ, Leveno KJ, Spong CY, Thom EA, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107(6):1226–32. DOI:10.1097/01.AOG.0000219750.79480.84 [ Links ]
11. Pilloni E, Alemanno MG, Gaglioti P, Sciarrone A, Garofalo A, Biolcati M, Botta G, Viora E, Todros T. The accuracy of ultrasound in antenatal diagnosis of placental attachment disorders. Ultrasound Obstet Gynecol. 2016 Mar;47(3):302-7. DOI:10.1002/uog.14893. [ Links ]
12. Warshak CR, Eskander R, Hull AD, Scioscia AL, Mattrey RF, Benirschke K, et al. Accuracy of ultrasonography and magnetic resonance imaging in the diagnosis of placenta accreta. Obstet Gynecol. 2006 Sep;108(3 Pt 1):573–81. DOI:10.1097/01. AOG.0000233155.62906.6d. [ Links ]
13. Maher MA, Abdelaziz A, Bazeed MF. Diagnostic accuracy of ultrasound and MRI in the prenatal diagnosis of placenta accreta. Acta Obstet Gynecol Scand. 2013 Sep;92(9):1017–22. DOI:10.1111/aogs.12187. [ Links ]
14. Rac MWF, Dashe JS, Wells CE, Moschos E, McIntire DD, Twickler DM. Ultrasound predictors of placental invasion: the Placenta Accreta Index. Am J Obstet Gynecol. 2015 Mar;212(3):343.e1–7. DOI:10.1016/j.ajog.2014.10.022. [ Links ]
15. Rahaim NSA, Whitby EH. The MRI features of placental adhesion disorder and their diagnostic significance: systematic review. Clinical Rad. 2015;70:917-25. DOI:10.1016/j. crad.2015.04.010. [ Links ]
16. Walker MG, Allen L, Windrim RC. Multidisciplinary management of invasive placenta previa. J Obstet Gynaecol Can. 2013;35:417-25. [ Links ]
17. Shetty MK, Dryden DK. Morbidly adherent placenta: ultrasound assessment and supplemental role of magnetic resonance imaging. Semin Ultrasound CT MRI. 2015;36;324-31. DOI:10.1053/j.sult.2015.05.007. [ Links ]
18. Eller AG, Porter TF, Soisson P, Silver RM. Optimal management strategies for placenta accreta. BJOG. 2011; 118:1084- 9. DOI:10.1111/j.1471-0528.2008.02037.x. [ Links ]
19. Warshak CR, Ramos GA, Eskander R, Benirschke K, Saenz CC, Kelly TF, et al. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Am J Obstet Gynecol. 2010;115: 65-9. DOI:10.1097/AOG.0b013e3181c4f12a. [ Links ]
20. Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. The management and outcomes of placenta accreta, increta, and percreta in the UK: a population-based descriptive study. BJOG. 2014;121:62-70. DOI:10.1111/1471-0528.12405. [ Links ]
21. Shamshiraz AA, Fox KA, Salmanian B, Diaz-Arrastia CR, Lee W, Baker BW, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9. DOI:10.1016/j.ajog.2014.08.019. [ Links ]
22. Silver RM , Fox KA, Barton JR, Abuhamad AZ, Simhan H, Huls CK, Belfort MA, Wright JD. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015 May;212(5):561-8. DOI:10.1016/j.ajog.2014.11.018. [ Links ]
23. Eller AG, Bennett MA, Sharshiner M, Masheter C, Soisson AP, Dodson M, Silver RM. Maternal morbidity in cases of placenta accreta managed by a multidisciplinary team compared with standard obstetric care. Obstet Gynecol. 2011;117:331- 7. [ Links ]
24. Stafford IA, Dashe JS, Shivvers SA, Alexander JM, McIntire DD, Leveno KJ. Ultrasonographic cervical length and risk of hemorrhage in pregnancies with placenta previa. Obstet Gynecol. 2010;116(3):595-600. DOI:10.1097/AOG.0b013e3181ea- 2deb. [ Links ]
25. Robinson BK, Grobman WA. Effectiveness of timing strategies for delivery of individuals with placenta previa and accreta. Obstet Gynecol. 2010;116(4):835-42. DOI:10.1097/ AOG.0b013e3181f3588d. [ Links ]
26. Gyamfi-Bannerman C, Thom EA, Blackwell SC, Tita AT, et al. Antenatal Betamethasone for women at risk for late preterm delivery. N Engl J Med, 2016;374(14):1311-20. DOI:10.1056/ NEJMc1605902. [ Links ]
27. Eller AG, Porter TF, Soisson P, Silver RM. Optimal management strategies for placenta accreta. BJOG. 2009;116:648-54. DOI:10.1111/j.1471-0528.2008.02037.x. [ Links ]
28. Wright JD, Siler RM, Bonanno C, Gaddipati S, Lu YS, Simpson LL, et al. Practice patterns and knowledge of obstetricians and gynecologists regarding placenta accreta. J Matern Fetal Neonatal Med. 2013;26:1602-9. DOI:10.3109/14767058.201 3.793662. [ Links ]
29. Ballas J, Hull AD, Saenz C, Warshak CR, Roberts AC, Resnik RR, et al. Preoperative intravascular balloon catheters and surgical outcomes in pregnancies complicated by placenta accreta: a management paradox. Am J Obstet Gynecol. 2012;207:216.e1-5. DOI:10.1016/j.ajog.2012.06.007. [ Links ]
30. Bishop S, Butler K, Monaghan S, Chan K, Murphy G, Edozien L. Multiple complications following the use of prophylactic internal iliac artery balloon occlusion in a patient with placenta percreta. Int J Obstet Anesth. 2011;20:70-3. DOI:10.1016/j. ijoa.2010.09.012. [ Links ]
31. Sentilhes L, Ambroselli C, Kayem G, Provansal M, Fernandez H, Perrotin F, et al. Maternal outcome after conservative management of placenta accreta. Obstet Gynecol. 2010;115:526-34. DOI:10.1097/AOG.0b013e3181d066d4. [ Links ]
32. Cunningham KM, Anwar A, Lindow SW. The recurrence risk of placenta accreta following uterine conserving management. J Neonatal Perinatal Med. 2015;8 (4):293-6. DOI:10.3233/ NPM-15915028. [ Links ]
33. Sentilhes L, Kayem G, Ambroselli C, Provansal M, Fernandez H, Perrotin F, et al. Fertility and pregnancy outcomes following conservative treatment for placenta accreta. Hum Reprod. 2010;25:2803-10. DOI:10.1093/humrep/deq239. [ Links ]
34. Pather S, Strockyj S, Richards A, Campbell N, de Vries B, Ogle R. Maternal outcome after conservative management of placenta percreta at cesarean section: a report of three cases and a review of the literature. Austr N Z J Obstet Gynaecol. 2014;54:84-7. DOI:10.1111/ajo.12149. [ Links ]
35. Clausen C, Lonn L, Langhoff-Roos J. Management of placenta percreta: a review of published cases. Acta Obstet Gynecol Scand. 2014;93:138-43. DOI:10.1111/aogs.12295. [ Links ]
Conflict of interest: The author has no conflicts of interest to report.
Recieved: August 20, 2016 Accepted: September 13, 2016
Correspondence address: 96 Jonathan Lucas Street Suite 634 Charleston, SC 29425