Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.62 no.4 Lima oct. 2016
SIMPOSIO EMERGENCIAS Y COMPLICACIONES SEVERAS OBSTÉTRICAS
Endocrine emergencies
Emergencias endocrinas
Christopher G. Goodier, MD1
1 Assistant Professor, Maternal Fetal Medicine, Obstetrics & Gynecology Department, Medical University of South Carolina, USA
Abstract
Endocrine emergencies such as thyroid storm and diabetic ketoacidosis should be considered life-threatening disease processes in the obstetric population. Diagnosis requires a high clinical suspicion with prompt initiation of treatment, supportive care and intervention. A multidisciplinary team of specialists, including maternal fetal medicine, endocrinology, medical intensivist, neonatologists and anesthesiology should be assembled to achieve the best out-comes for mother and baby.
Keywords: Pregnancy; Diabetes; Diabetic Ketoacidosis; Thyroid Dysfunction; Hyperthyroidism; Thyroid Storm.
Resumen
Las emergencias endocrinas, tales como la tormenta tiroidea y la cetoacidosis diabética, deben ser consideradas como procesos mórbidos que ponen en riesgo la vida de la población obstétrica. El diagnóstico requiere gran sospecha clínica e inicio inmediato del tratamiento, soporte clínico e intervención. Se debe organizar un equipo multidisciplinario de especialistas que incluyan la medicina maternofetal, endocrinología, intensivista médico, neonatólogos y anestesiólogos, de manera de lograr el mejor resultado para la madre y el bebe.
Palabras clave: Embarazo; Diabetes; Diabetes, cetoacidosis; Tiroides, disfunción; Hipertiroidismo; Tiroides, Tormenta.
Thyroid storm
Hyperthyroidism occurs in 0.1-0.4% of all pregnancies and usually the result of maternal Grave’s disease, an autoimmune condition characterized by the production of thyroid stimulating immunoglobulin (TSI) and TSH binding inhibitory immunoglobulin(1). Early treatment and normalization of maternal thyroid function important because poor control increases the risk of PTB, fetal morbidity and thyroid crisis.
Thyroid storm is a potentially fatal endocrine emergency characterized by a severe hyper-metabolic state precipitated by high levels of endogenous thyroid hormones. While the exact triggering mechanism is unknown, most cases are a result of poorly controlled hyperthyroidism. It has been associated with preceding events such as infection, surgery, trauma, venous thromboembolism and diabetic ketoacidosis.
Case fatality rates vary in different series, but range from 10-30%(2); however with treatment incidence in pregnancy 2%(3). Rapid recognition, prompt supportive care and intervention likely maximizes maternal and fetal outcome.
Presenting signs and symptoms can be variable, and may commonly include tachycardia, diaphoresis, anxiety or confusion, weakness, hyperpyrexia and a widened pulse pressure(4). The presentation may be nonspecific enough to be confused with any number of other conditions, leading to a delay in diagnosis and treatment. A prominent goiter, thyroid bruit or exopthalmos may be discriminating physical signs that are more specific to thyroid dysfunction.
Laboratory analysis may be useful in making a diagnosis, especially in patients who have no reported history of thyroid dysfunction. Thyroid stimulating hormone (TSH) is usually undetect able, although in the first trimester this may be due to the normal physiology of pregnancy due to the suppressive effects of human chorionic gonadotropin (hCG). Free T4 (FT4) and free T3 (FT3) are often well above the upper limits of normal in pregnancy. Total hormone levels are also usually elevated. Trimester and laboratory specific reference ranges should be used; although typically the goal FT4 is set at the upper limits of the normal range. However there are no generally accepted levels at which the diagnosis of thyroid storm is assured, and there may be significant laboratory overlap with simple hyperthyroidism. The white blood cell count (WBC) may be variably elevated, especially if there is an associated infection. There may also be evidence of dehydration, acidosis, hyperglycemia, hypercalcemia, elevated liver enzymes and electrolyte disturbances on metabolic panel screening.
While thyroid storm is primarily a clinical diagnosis, Burch and Wartofsky have outlined a commonly cited clinical scoring system for the probability of thyroid storm(5). Points are allocated for elevation in temperature, maternal pulse and a number of organ system dysfunctions. Scores can indicate a high, medium or low probability of the diagnosis of thyroid storm. Chest X-rays and CT scans can be useful when looking for concomitant infections or pulmonary pathology such as edema or emboli.
The maternal adaptations to pregnancy are also factors that must be considered when making the diagnosis of thyroid storm. Mild tachycardia and leukocytosis are not unusual in pregnancy. Pre-eclampsia may present with hypertension, systemic symptoms, pulmonary edema and even heart failure. Interpretation of maternal acid-base status must take into consideration the moderate compensated respiratory alkalosis that occurs in pregnancy.
Fetal heart rate tracings may reflect maternal metabolic changes, including fetal tachycardia (>160 beats per minute), loss of variability and in severe cases late decelerations, suggesting hypoxia and acidosis. Fetal morbidity remains high with thyroid storm, with significant incidences of preterm birth, neonatal complications and even fetal death(6).
A diagnosis of thyroid storm requires prompt intervention for both mother and fetus. Multidisciplinary care should include Maternal-Fetal Medicine, Endocrinology and possibly Critical Care specialists, as ICU admission may be necessary. Once viability is achieved, continuous fetal monitoring should be available. The presence of a persistent fetal bradycardia or the development of Category III heart rate tracing that is unresponsive to resuscitative measures may require expedited delivery for a viable fetus. Generally speaking, the maternal condition should be treated and stabilized; prior to intervening on behalf of the fetus(7).
Treatment should include 1) adequate intravenous access; 2) replacement of fluids; 3) interpretation of heart rhythm; and, 4) ensuring oxygenation. EKG, continuous monitoring with telemetry if possible and pulse oximeter should be placed. Laboratory evaluation should include TSH, FT3/4, liver and kidney function as well as electrolytes and blood count with differential. Respiratory failure is a late finding, but intubation and mechanical ventilation is sometimes necessary.
Definitive treatment of thyroid storm also involves the use of several medications in addition to supportive care. First-line treatment of hyperthyroidism is the thionamides, specifically propylthiourocil (PTU) and methimazole (MMI) (8). Both agents act within the thyroid gland to inhibit follicular growth and development. One advantage of PTU is that it also works outside the thyroid gland to inhibit peripheral conversion at the tissue level of T4 to T3. PTU how ever is not typically first line given that there have been rare cases of fulminate liver failure and death associated with its use, including instances in pregnancy. There is a FDA "black box" warning for PTU concerning this link to hepatotoxicity. It is unclear how thyroid storm specifically affects this risk.
Methimazole use in pregnancy has been linked to some teratogenic effects including aplasia cutis(9). This association is not particularly strong in the literature so its use is generally considered to be acceptable, especially outside the time of organogenesis. In addition, rarely a life-threatening agranulocytosis may develop after MMI use. Given these conflicting risks there is no clear recommendation for which thionamide to initiate in a thyroid storm.
In addition to PTU or MMI to treat thyroid storm, it is also recommended to use iodide-containing medication to inhibit the further release of active thyroid hormone from the thyroid gland. Oral potassium iodide, 5 drops every 8 hours, or IV sodium iodide 1 000 mg every 12 hours may be used. The use of iodines should not be administered prior to the use of thionamides due to their initial action, which releases thyroid hormones from the thyroid, prior to suppressing it. The general time frame is recommended to be 30-60 minutes after the initiation of thionamides(8).
Corticosteroids can also be administered which have the effect of decreasing systemic inflammation as well as peripheral effects of decreasing T4 to T3 conversion. Other supportive medications again include antipyretics such as acetaminophen and beta-blockade.
Beta blockade is a critical adjunct to treatment of thyroid storm and tachycardia leading to high output cardiac failure is one of the leading causes of mortality in a thyroid storm. Propranalol may be used for rate control and has the advantage of reduces peripheral T4 to T3 conversion. Long-term use of beta-blockers has been associated with fetal growth restriction, but is generally considered safe in a risk/benefit consideration. Atenolol is generally avoided (Category D).
If conventional treatments fail to generate an adequate response, emergency thyroidectomy, with or without plasmaphoresis has been described successfully in thyroid storm, but must be considered a high-risk undertaking(10).
Diabetic ketoacidosis
Diabetic ketoacidosis (DKA) is a medical emergency and can result in both maternal and fetal morbidity and mortality with and overall incidence, which has decreased, from approximately 10 to 20% in the late 1970s to approximately 1 to 2% in most recent reports(11-13). The improvement has been attributed to early recognition and aggressive multi-disciplinary management. As a result both maternal and fetal mortality have significantly improved. Preterm birth, both from premature labor and from medical intervention, is a common sequalae from severe DKA.
The pathophysiology of DKA is the result of a complex interplay in which inadequate insulin action results in perceived hypoglycemia at the cellular level of target cells such as those in the liver, adipose and muscle tissues. The body responds by releasing glucagon, which worsens the level of hyperglycemia in the serum causing osmotic diuresis and resultant hypovolemia and electrolyte depletion.
The lack of insulin and production of counter-regulatory hormones in the adipose tissue activates hormone sensitive lipase, which causes the release of free fatty acids into the circulation. The free fatty acids are then oxidized to ketone bodies. Acidosis occurs secondary to ketone body formation, which can overwhelm the buffering capacity of the body leading to a metabolic acidosis manifested as an anion gap. Ketoacids bind sodium and potassium, which are excreted in the urine, further worsening the electrolyte balance. The final common pathway if this process is left untreated can lead to cardiac dysfunction, decreased tissue perfusion and worsened real function leading to shock, coma and death(11,16).
The normal physiologic changes of pregnancy in-crease the susceptibility of the gravid parturient to DKA. Pregnancy related physiology results in increased insulin resistance primarily due to the action of human placental lactogen. Thus, normal exogenous insulin requirements increase with advancing gestation.
In addition, respiratory adaptations of normal pregnancy produce a compensated maternal respiratory alkalosis. The compensatory decrease in serum bicarbonate reduces the body’s normal buffering capacity, thus predisposing the patient to DKA(11,12,16).
Patients generally present with abdominal pain, malaise, persistent vomiting, polydypsia, hyperventilation, tachycardia, dehydration and polyuria. As the level of acidosis increases patient will often have altered mental status and sometimes present obtunded. The diagnosis is confirmed with documentation of hyperglycemia, acidosis and ketonuria. Other laboratory findings include anion gap, ketonemia, renal dysfunction as well as possible electrolyte abnormalities(12,16).
Typically patient presents with serum glucose levels greater than 300 mg/dL; however this threshold is much lower in pregnancy and DKA can occur with levels <200 mg/dL(13).
Precipitating factors for diabetic ketoacidosis include emesis, infection, previously undiagnosed diabetes, beta-sympathomimetic tocolytic agents, corticosteroids, poor compliance, pump malfunctions and medical errors(14,15). While beta-sympathomimetics (eg. terbutaline) are not routinely used for prolonged (>48 hrs) tocolysis due to the FDA safety communication in 2011 warning potential for serious maternal heart problems and death, it is important to remember that they should be used very cautiously, if ever, for patients with diabetes (11).
Maternal DKA presents a significant risk to overall fetal well being. The mechanism is not clearly understood; however appears to be related to ketoacid production, which readily cross the placenta, decreasing fetal tissue perfusion and reducing oxygenation in the fetus. In addition these ketoacids can dissociate into anions and result in fetal metabolic acidosis(16). The fetus has a limited ability to buffer significant acidemia, and therefore is quite sensitive to maternal acidosis.
This often results in a fetal heart tracing that may include decreased variability or late decelera tions. Interpretation can be difficult; however, it is likely that maternal acidosis, hyperglycemia and hypovolemia all contribute to a potential threat to fetal well-being(12). Initially, the primary effort should focus on correcting the maternal acidosis, which should improve the underlying fetal status. It is important to exhaust all attempts to correct the underlying maternal abnormalities prior to intervening for the fetus(13,16).
Like thyroid storm, DKA is considered a medical emergency and a multidisciplinary team should be assembled, including Maternal-Fetal-Medicine, Neonatology and Critical Care, as soon as possible. In addition, strong consideration should be made for admission to an intensive care unit.
Treatment includes adequate IV access and placement of an indwelling urinary catheter. Significant fluid deficits should be anticipated and corrected, insulin should be started and electrolyte abnormalities corrected. Fluid balance and vital signs need to be carefully monitored and documented.
Basic labs, including a complete metabolic panel with magnesium and phosphorous, complete blood count with differential, urinalysis, fingerstick blood glucose, arterial blood gas and serum ketones should be collected. Additional testing (urine culture, blood culture, chest X ray, etc.) should be performed based on clinical suspicion and any potential underlying processes. Initially, urine/serum ketones, electrolytes and maternal acid/base status should be monitored every 2 hours until ketosis and acidosis resolved. Blood sugars should be collected hourly during this time to titrate appropriate insulin doses (11,12,16-18).
Once viability is confirmed, fetal monitoring should be initiated. As noted, the fetal heart tracing will likely appear concerning during the initial insult. Maternal oxygen supplementation and favorable maternal positioning should be employed to increase blood flow to fetus and improve oxygenation. Adequate hydration and correction of acid/base derangements must be started. Delivery should usually be postponed until after maternal metabolic condition is stabilized, as this will usually correct the fetal heart tracing abnormality. There are exceptions, including intractable bradycardia or persistent Category III tracings.
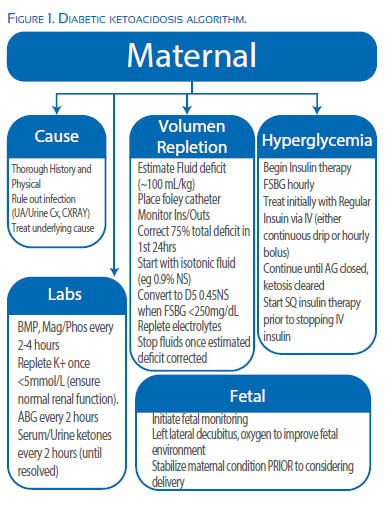
Figure 1 illustrates the goals of treatment for any patient with diabetic ketoacidosis, including rehydration, reduction of hyperglycemia, correction of acid-base and electrolyte imbalance while searching for and treating the underlying etiology(11,12,16-18).
The fluid deficit leading to the profound intravascular depletion is estimated at 100 mL/kg of total body weight and is typically 4-10 liters(19). Initially, aggressive intravenous replacement with isotonic normal saline should be started with the goal to replace approximately 75% of the overall deficit within the first 24 hours. Hypotonic fluids (e.g. lactated ringers and 0.45% saline) should be avoided initially as they can cause a rapid decline in plasma osmolarity leading to cerebral edema. Blood glucose values should be monitored hourly as they will decrease with initial hydration alone. Once the serum glucose reaches <250 mg/dL fluids should be switched to D5-0.45% normal saline.
Intravenous insulin administration should be undertaken immediately to aid in lowering blood glucose levels associated with lipolysis and ketogenesis. Subcutaneous and intramuscular are typically avoided due to the slower onset of action, which is worsened in DKA 12. The initial blood glucose target is 150-200 mg/dL in order to avoid rapid correction and resulting complications.
It is important to continue the insulin infusion until the anion gap is closed and acidosis resolved. This can take significantly longer than correcting the hypoglycemia and typically takes 12-24 hours.
It is important to remember that insulin requirements can be significant and most protocols suggest an initial bolus dose of 10-20 units of regular insulin, followed by an infusion rate of 5-10 units/hour. This amount should be increased if the blood glucose values do not fall 25-20% over two hours. Once it is deemed safe to transition to subcutaneous insulin, the first dose should be given prior to the discontinuation of the intravenous infusion to decrease the risk of recurrent ketoacidosis.
Potassium is the most common electrolyte abnormality in DKA. The levels are often normal at the time of initial DKA diagnosis; however the actual deficit is estimated at 5-10meq/kg and replacement should begin after fluid and insulin therapy has begun, as well as adequate renal function established (i.e. do not start until you ensure appropriate urine output). The goal is to maintain potassium levels between 4-5mmol/L and is not begun until the serum potassium falls below 5. Potassium chloride is typically used and can either be added to the maintenance IV fluids of repleted separately. Serum potassium levels should be checked every 2-4 hours as significant hypokalemia can precipitate a cardiac arrhythmia.
Replacement of low serum bicarbonate levels remain a source of controversy and replacement is generally agreed upon if the patients pH is <7.0. Some studies have shown that routine replacement of low serum levels of bicarbonate have not proven beneficial in DKA and may cause unnecessary maternal and fetal complications. Replacement can delay the correction of ketoacids in the maternal bloodstream and, if corrected to rapidly, elevate fetal PCO2 impairing fetal ability to maintain adequate O2 transfer (11,18).
Referencias bibliográficas
1. De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. Summary: Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab, August 2012;97(8):2543–65. DOI: 10.1210/jc.2011-2803. [ Links ]
2. Tietgens ST, Leinung MC. Thyroid storm. Med Clin North Am. 1995;79:169-84. [ Links ]
3. Davis LE, Lucas MJ, Hankins, GD, Roark ML, Cunningham FG. Thyrotoxicosis complicating pregnancy. Am J Obstet Gynecol. 1989 Jan;160(1):63-70. [ Links ]
4. Nayak B, Burman K. Thyrotoxicosis and thyroid storm. Endocrinol Metab Clin N Am. 2006;35:663-86. DOI:10.1016/j. ecl.2006.09.008. [ Links ]
5. Burch HB, Wartofsky L. Thyroid storm. Endocrinol Metab Clin North Am. 1993;22:266-77. [ Links ]
6. Delport EF. A thyroid-related endocrine emergency in pregnancy. JEMDSA. 2009;14(2):99-101. [ Links ]
7. Rashid M, Rashid MH. Obstetric management of thyroid disease. Obstet Gynecol Surv. 2007;62(10):680-8. DOI:10.1097/01.ogx.0000281558.59184.b5. [ Links ]
8. Bahn RS, Burch H, Copper DS, Garber JR, Greenlee MC, Klein I, Laurberg P, McDougall IR, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and the American Association of Clinical Endocrinologists. Endocr Pract. 2011 May- Jun;17(3):456-520. [ Links ]
9. Gianantonio E, Schaefer C, Mastroiacovo P. Adverse fetal effects of prenatal methimazole exposure. Teratology. 2001;64(5):262-6. DOI: 10.1002/tera.1072. [ Links ]
10. Vyas AA, Vyas P, Fillipon NL, Vijayakrishnan R, Trivedi N. Successful treatment of thyroid storm with plasmaphoresis in a patient with MMI-induced agranulocytosis. Endocr Pract. 2010 Jul-Aug;16(4):673-6. DOI:10.4158/EP09265.CR. [ Links ]
11. Parker JA, Conway DL. Diabetic ketoacidosis in pregnancy. Obstet Gynecol Clin N Am. 2007;34:533-43. DOI: 10.1016/j. ogc.2007.08.001. [ Links ]
12. Abdu TAM, Barton DM, Baskar V, Kamalakannan D. Diabetic ketoacidosis in pregnancy. Postgrad Med J. 2003:79(934):454- 7. [ Links ]
13. Whiteman VE, Homko CJ, Reece EA. Management of hypoglycemia and diabetic ketoacidosis in pregnancy. Obstet Gynecol Clin. 1996;23(1):88-107. [ Links ]
14. Rogers BD, Rogers DE. Clinical variable associated with diabetic ketoacidosis during pregnancy. J Reprod Med. 1991;36:797-800. [ Links ]
15. Montoro MN, Myers VP, Mestman JH, Xu Y, Anderson BG, Golde SH. Outcome of pregnancy in diabetic ketoacidosis. Am J Peritanol. 1993 Jan;10(1):17-20. DOI: 10.1055/s-2007- 994692. [ Links ]
16. Carroll M, Yeomans ER. Diabetic ketoacidosis in pregnancy. Crit Care Med. 2005;33:S347-53. [ Links ]
17. Ramin K. Diabetic ketoacidosis in pregnancy. Obstet Gynecol Clin. 1999;26(3):481-8. [ Links ]
18. Foley MR, Strong TH, Garite TJ. Obstetric Intensive Care Manual. 2nd ed. McGraw Hill Publishers; p.143-154. [ Links ]
19. Chauhan SP, Perry KG, McLaughlin BN, Roberts WE, Sullivan CA, Morrison JC. Diabetic ketoacidosis complicating pregnancy. J Perinatol. 1996;16(1):173-5. [ Links ]
Conflict of interest: I have no conflict of interest
Recieved: August 20, 2016 Accepted: September 29, 2016
Correspondence: Christopher G. Goodier, MD 96 Jonathan Lucas St, CSB 634 MSC 619 Charleston, SC 29425 Tel 843 792 4500 Fax 843 792 0533