Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.2 Lima Apr-Jun 2020
http://dx.doi.org/10.31403/rpgo.v66i2252
Case Report
Placental massive perivillous fibrinoid deposition causing severe intrauterine growth restriction
1. Daniel Alcides Carrión National Hospital, Callao, Peru
a. Obstetrician/gynecologist, Daniel Alcides Carrión National Hospital, Callao, Peru; Center for Fetal Medicine-CENMEF. Maternal-Fetal Medicine Group - Fetalis
b Pathologist, Daniel Alcides Carrión National Hospital, Callao, Peru; Arias Stella Pathology and Molecular Biology Institute
Placental massive perivillous fibrinoid deposition or maternal floor infarction is a rare entity associated with intrauterine growth restriction, fetal death and poor perinatal outcome. It is characterized by the perivillous deposition of fibrinoid material, without a clear etiology. We present the first documented case in Peru.
Key words: Placenta; perivillous fibrin deposition; Fetal growth restriction
Introduction
Massive perivillous fibrinoid deposition or maternal floor infarction is a rare condition occurring in 0.3 to 0.5% of all pregnancies1. The exact pathophysiological mechanism of this "fibrinoid" deposition around chorionic villi is unknown, but diverse etiologies have been proposed: infections, autoimmune diseases (lupus), antiphospholipid syndrome, maternal coagulopathy, angiogenic-antiangiogenic disequilibrium and chronic intervillositis1-3. Most series are retrospective and study the placenta in relation to intrauterine growth restriction, fetal death and poor perinatal outcome. We present the first case report in our country of severe intrauterine growth restriction with sonographic placental abnormalities and the definitive pathology diagnosis of massive perivillous fibrinoid deposition.
Case report
A primiparous patient, 36 weeks and 2 days pregnant by early ultrasound, was evaluated in the emergency room for decreased fetal movements. Her fundal height was 27 cm.
The following measurements were obtained on the ultrasound scan: biparietal diameter (BPD) 84 mm (4th percentile), head circumference (CC) 308 mm (p<3), abdominal girth (AG) 281 mm (p<3), femur length (FL) 59 mm (p<3), average fetal weight 1 921 g ±284 g, below the 3rd percentile for gestational age (GA); the ultrasound also revealed oligohydramnios. Further findings were anterior placenta located in the uterine body, heterogeneous and thick (67 mm), with multiple hyperechoic areas either in the basal plate or throughout its thickness, and hypoechoic areas in the center of the cotyledon, suggesting perivillous thrombosis (Figure 1). On the Doppler ultrasound, the umbilical arterial pulsatility index (PI) was 1.26 (appropriate for GA [AGA]), the middle cerebral artery pulsatility index was 0.92 (altered, below the 5th percentile for GA) and the peak systolic velocity was 72 cm/s (greater than 2.5 multiples of the median [MoM] for GA), venous duct PI was 0.61 and the average PI of the uterine arteries was 0.8 (AGA). The patient was diagnosed with late fetal growth restriction, with signs of flow redistribution and placentomegaly.
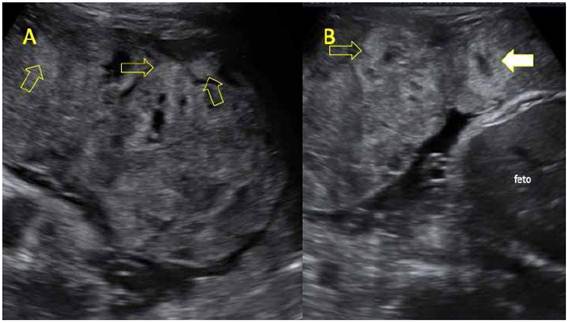
Figure 1 A-B. UltrAsound Ultrasound image of heterogeneous placenta. the yellow arrows point at hyperechoic areas in the maternal surface, corresponding to areas of massive perivillous fibrinoid deposition. white arrow: central hypoechoic area in the cotyledon, suspicious for intervillous thrombus.
A female newborn was delivered through an emergency C-section, weighing 2 155 g, with a height of 44 cm and an Apgar score of 8 and 9 at minutes 1 and 5, respectively. At macroscopic examination, most placental cotyledons were yellowish grey in the maternal surface and yellowish in the fetal surface. Serial sectioning revealed the maternal surface was whitish yellow, with variable thickness, expanding to the fetal surface in some areas, forming irregular tracts. Some cotyledons had perivillous thrombi (Figures 2 and 3). Upon macroscopy and microscopy, a diffuse deposition of fibrinoid material was noted in more than 50% of the villi, extending from the maternal into the fetal surface. Some areas also had perivillous thrombi (Figure 4). The newborn progressed favorably, without requiring advanced life support, and was discharged from the neonatology service 7 days later.
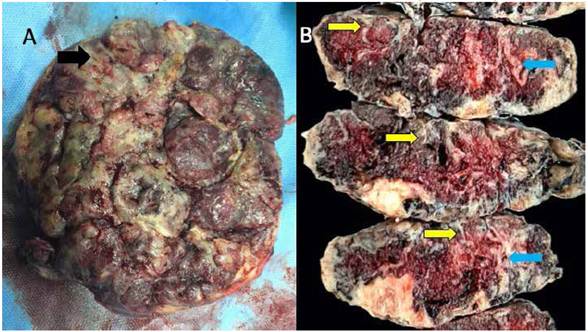
Figure 2 A. Image of the maternal surface of the placenta showing the yellowish gray color of compromised cotyledons, with areas of massive perivillous fibrinoid deposition (black arrow). b. serial macroscopic sections of the placenta, all of them showing a yellowish band of varying thickness (yellow arrow), compromising all the width in some areas (light blue arrow).
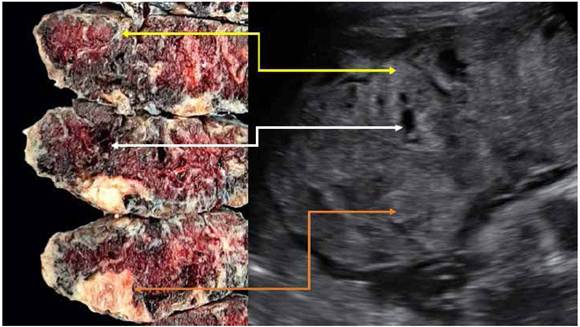
Figure 3 Relation between pathology and ultrasound findings. yellow arrow: hyperechoic area on the ultrasound scan corresponds to perivillous fibrinoid deposition (maternal floor infarction). white arrow: hypoechoic area corresponds to perivillous thrombosis. orange arrow: hyperechoic area close to the fetal surface corresponds to placental infarction.
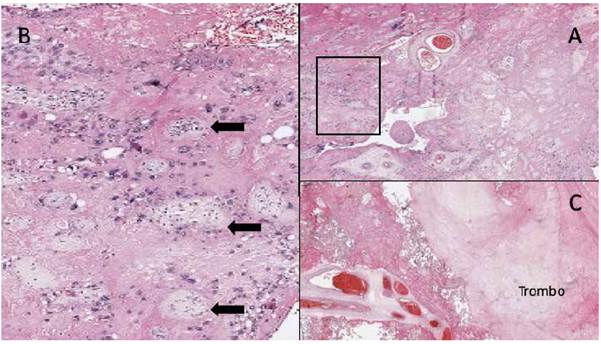
Figure 4 A. Full slide image showing perivillous fibrin extending from the maternal into the fetal surface, compromising ≥50% of villi in ≥ 1 slide. b. enlargement of a, where chorionic villi (black arrows) are surrounded by abundant fibrinoid material. c. image of a perivillous thrombus surrounded by chorionic villi with fibrin.
Discussion
The concept of maternal floor infarction (MFI) was proposed by Benirschke and Driscoll4 to describe the deposition of fibrinoid material-it is not really a vascular occlusive phenomenon. Afterwards, Fox5 defined massive perivillous fibrinoid deposition (MPFD) as a fibrin deposition compromising most of the placenta; this deposition can be transmural. Both terms have been used in a similar way in the literature, as part of the spectrum of a single disease. Katzman2 proposed a semiquantitative classification: maternal floor infarction (fibrinoid material in the maternal surface, over 3 mm thick), transmural or severe MPFD (fibrinoid material extends from the maternal into the fetal surface, compromising over 50% of the villi in one slide), borderline or moderate MPFD (fibrinoid material extends transmurally or quasi-transmurally, compromising 25 to 50% of the villi in one slide), and non-classifiable disease.
Diagnosis is obtained through histology. At macroscopy, the placental maternal surface is whitish, grayish or yellowish. Serial sections of the placenta show irregular and sinuous white yellowish areas, confined to the maternal surface or piercing through all of the placental thickness3; these characteristics were clearly observed in the case we report (Figures 2 and 3). Microscopic examination reveals the perivillous deposition of a "fibrinoid" substance closing the space without collapsing it, surrounding viable and atrophic chorionic villi. This fibrinoid substance consists of a mixture of blood proteins such as fibrin, basal membrane collagen, fibrinogen, fibronectin and laminin. Figure 4 shows the slide that established the definitive histopathological diagnosis in our case. This condition is frequently associated with perivillous thrombosis, ischemic villitis, plasma cell deciduitis and villitis of uncertain significance2,3,6,7.
The placental ultrasound may show heterogeneous hyperechoic areas in the maternal surface that might extend to the fetal surface, usually with increased placental thickness8,9. These hyperechoic areas are showed in Figure 1. Frequently, these findings present with other images suggestive of perivillous thrombosis and placental infarction8,9. Figure 3 links ultrasound findings to placental macroscopy, showing that heterogeneous hyperechoic areas correspond with perivillous fibrinoid deposition.
The most frequent clinical presentation is intrauterine growth restriction (IUGR), with an incidence between 31 and 100%. It may be of early or late onset and, on some occasions, present with alterations in the fetal or maternal Doppler ultrasound. Table 1 summarizes the main series that relate MPFD to IUGR. Devisme11 found 71 cases of MPFD among 6971 placentas, which he classified as severe (50% of the villi were compromised in one slide) or moderate (25 to 50% of the villi were compromised). The incidence of IUGR was 93 and 82% in each group respectively, with Doppler alterations of the uterine arteries in half of the cases. 60% of the cases with Doppler alterations of the uterine artery had severe MPFD, and 25% had moderate MPFD. Spinillo6, in a prospective series of 355 fetuses with IUGR, obtained a global MPFD incidence of 11.8% (incidence of 3.1% for severe and of 8.7% for moderate cases). Deterioration in fetal Doppler ultrasound findings was more frequent in severe cases, mainly due to an alteration of the pulsatility index (36% vs 22%); this deterioration did not evidence absent nor reversed end-diastolic flow. Vasodilation of the middle cerebral artery was more frequent in severe cases (54% vs 25%). These results show that placental insufficiency would not be the only explanation for an adverse perinatal outcome; the concomitance of placental vascular alterations and inflammation could also explain this phenomenon. Studying the placentas of 54 cases compatible with MPFD and IUGR, Spinillo14 found that they were at a higher risk for alterations of the umbilical artery in the Doppler ultrasound (OR 1.2, PI over the 95th percentile, 2.1 for absent or reversed diastole), without further Doppler compromise in relation to the severity of MPFD. In the Doppler assessment of the MCA of the case we report, we found vasodilation and an altered cerebroplacental index, evidencing flow redistribution in relation to placental insufficiency. It is worth noting that the increase in the peak systolic velocity of the MCA still has no clear explanation.
Table 1 Published case series of massive perivillous fibrinoid deposition in relation to intrauterine growth restriction.
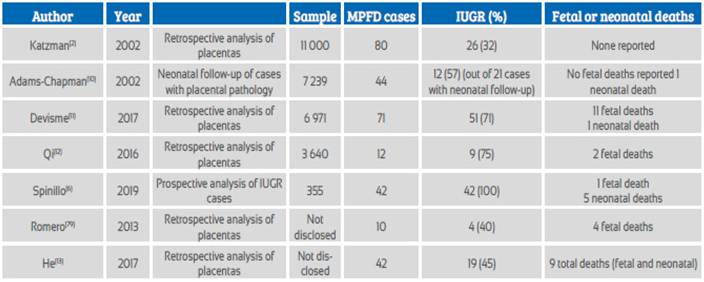
Another frequent clinical presentation is fetal death, with a reported incidence of 17 to 50%2,11,15 (see Table 1). In a study of 575 placentas from cases of fetal death, Man16 identified placental pathologies in 19%, of which 10% were MPFD, most of which belonged to third trimester fetal deaths. Similarly, Devisme11 reports an incidence of fetal deaths of 9% in cases of severe MPFD and of 2% in cases with moderate disease.
A very important issue in the management of MPFD is the risk of recurrence in the following pregnancies, which may be up to 30%2-13. Because of this, studying the placenta of a fe-tus with IUGR is critical for future pregnancies. Some case reports mention treatments, such as aspirin or low-molecular-weight heparin, that were used in specific patients who had no recurrent disease in subsequent pregnancies, and gave birth to children with good perinatal out comes13,17.
REFERENCES
1. Katzman PJ, Ernst LM, Scheimberg IB. Massive perivillous fibrinoid deposition and maternal floor infarct. In: Khong TY. Pathology of the Placenta, 2019;77-82. Doi: 10.1007/978-3-319-97214-5_8 [ Links ]
2. Katzman PJ, Genest DR. Maternal floor infarction and massive perivillous fibrin deposition: histological definitions, association with intrauterine fetal growth restriction, and risk of recurrence. Pediatr Dev Pathol 2002;5:159-64.Doi: 10.1007/s10024001-0195-y [ Links ]
3. Faye-Petersen OM, Ernst LM. Maternal floor infarction and massive perivillous fibrin deposition. Surg Pathol Clin. 2013;101-14. Doi 10.1016/j.path.2012.10.002 [ Links ]
4. Fox H, Elston CW. Pathology of the placenta. Major Probl Pathol. 1978;7:1-491. [ Links ]
5. Benirschke K, Driscoll SG. Maternal floor infarction. In: Benirschke K, Driscoll SG, eds. Pathology of the Human Placenta. New York: Springer-Verlag, 1967;328-30. [ Links ]
6. Spinillo A, Gardella B, Muscettola G, Cesari S, Fiandrino G, Tzialla C. The impact of placental massive perivillous fibrin deposition on neonatal outcome in pregnancies complicated by fetal growth restriction. Placenta. 2019;87:46-52. DOI: 10.1016/j.placenta.2019.09.007 [ Links ]
7. Romero R, Whitten A, Korzeniewski SJ, Than NG, Chaemsaithong P, Miranda J, et al. Maternal floor infarction/ massive perivillous fibrin deposition: a manifestation of maternal antifetal rejection? Am J Reprod Immunol. 2013;70(4):285-98. doi:10.1111/aji.12143 [ Links ]
8. Kingdom JC, Audette MC, Hobson SR, Windrim RC, Morgen E. A placenta clinic approach to the diagnosis and management of fetal growth restriction. Am J Obst Gynecol. 2018;218(2S):S803-S817. Doi: 10.1016/j.ajog.2017.11.575 [ Links ]
9. Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018;218(2S):S745-S761. doi: 10.1016/j.ajog.2017.11.577 [ Links ]
10. Adams-Chapman I, Vaucher YE, Bejar RF, Benirschke K, Baergen RN, Moore TR. Maternal floor infarction of the placenta: association with central nervous system injury and adverse neurodevelopmental outcome. J Perinatol. 2002;22(3):236-41. DOI: 10.1038/sj/jp/7210685 [ Links ]
11. Devisme L, Chauvie`re C, Franquet-Ansart H, Chudzinski A, Stichelbout M, Houfflin-Debarge V, et al. Perinatal outcome of placental massive perivillous fibrin deposition: a case-control study. Prenat Diagn. 2017;37(4):323-8 doi: 10.1002/pd.5013 [ Links ]
12. Qi M, En Chang KT, Quan Lian DW, Khoo CK, Tan KH. Placental massive perivillous fibrinoid deposition is associated with adverse pregnancy outcomes: a clinicopathological study of 12 cases. Case Rep Perinat Med. 2016;5(1):35-9. DOI 10.1515/crpm-2015-0087 [ Links ]
13. He M, Migliori A, Maari NS, Mehta ND. Follow-up and management of recurrent pregnancy losses due to massive perivillous fibrinoid deposition. Obstet Med. 2018;11(1):17-22. doi: 10.1177/1753495X17710129. [ Links ]
14. Spinillo A, Gardella B, Bariselli S, Alfei A, Silini E, Dal Belo B. Placental histopathological correlates of umbilical artery Doppler velocimetry in pregnancies complicated by fetal growth restriction. Prenat Diagn. 2012;32:1263-72. DOI: 10.1002/pd.3988 [ Links ]
15. Mandsager NT, Bendon R, Mostello D, Rosenn B, Miodovnik M, Siddiqi TA. Maternal floor infarction of the placenta: prenatal diagnosis and clinical significance. Obstet Gynecol. 1994;83,750-4. [ Links ]
16. Man J, Hutchinson JC, Heazell AE, Ashworth M, Jeffrey I, Sebire NJ. Stillbirth and intrauterine fetal death: role of routine histopathological placental findings to determine cause of death. Ultrasound Obstet Gynecol. 2016;48:579- 84. DOI: 10.1002/uog.16019 [ Links ]
17. Abdulghani S, Moretti F, Gruslin A, Grynspan D. Recurrent massive perivillous fibrin deposition and chronic intervillositis treated with heparin and intravenous immunoglobulin: a case report. J Obstet Gynaecol Can. 2017;39(8):676-81. Doi: 10.1016/j.jogc.2017.03.089 [ Links ]
Received: February 02, 2020; Accepted: February 04, 2020











 text in
text in 


