Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.2 Lima abr-jun 2020
http://dx.doi.org/10.31403/rpgo.v66i2253
Case Report
Invasive hydatidiform mole coexistent with normal fetus. Case report
1. Hospital Nacional Alberto Sabogal Sologuren, Callao, Perú
We report the case of a pregnant woman referred to our hospital for suspected partial hydatidiform mole. Ultrasound images showed a normal fetus attached to a small placenta adjacent to a honeycomb-like tumor mass. Amniocentesis revealed a normal karyotype. Due to β-hCG values greater than 800 000 IU and a mass growth of 11% by magnetic resonance imaging, an ultrasound-guided percutaneous tumor biopsy was performed; it ruled out the possibility of choriocarcinoma. The patient had symptoms of hyperthyroidism that required treatment; when the β-HCG levels exceeded one million IU, a course of chemotherapy was prescribed. At 29 weeks, the patient started labor; a cesarean hysterectomy was performed, obtaining a live newborn with Apgar 5 and 7. The pathology report informed the placental mass as an invasive mole. According to our literature search, this is the first case report where an invasive mole coexisted with a healthy fetus. We highlight the importance of using all diagnostic and management tools necessary to achieve fetal viability, without increasing the maternal risk of complications.
Key words: Pregnancy; Hydatidiform mole; invasive; Fetus
Introduction
The World Health Organization classifies gestational trophoblastic disease (GTD) as a disorder of placental development (complete, partial and invasive hydatidiform mole) and trophoblastic tumors (choriocarcinoma, trophoblastic tumor of the placental site and epithelioid trophoblastic tumor), among others1.
The coexistence of a complete mole and a healthy twin is a rare event, with a prevalence of 1 in 20 000 to 100 0002; the probability of obtaining a healthy newborn varies from 7 to 40%3,4. This low rate is due to maternal fetal complications such as spontaneous abortion, preterm delivery, intrauterine fetal death, bleeding, preeclampsia, persistent trophoblastic disease and thyrotoxicosis5. We report the case of a patient referred to our hospital and the management strategy used to achieve neonatal viability.
Case report
A 38-year-old patient, 20 3/7 weeks pregnant, was admitted to our hospital referred from the city of Huaral, Lima, Peru, due to hydatidiform mole. She had suffered from pelvic pain for the last two months, without bleeding nor amniotic fluid leakage. Gravida 3 para 2, both eutocic deliveries, the patient had no contributing history.
Ultrasound examination revealed a 490 g fetus (35th percentile) with an anterior placenta, adjacent to a honeycomb tumor covering the inferior uterine region, including the internal os; no flow was captured in the Doppler velocimetry (Figure 1).
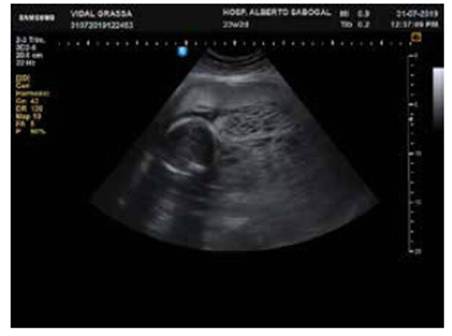
Figure 1 Ultrasound showing a healthy fetus, a normal placenta in the upper anterior side and an adjacent tumor with a "snowstorm" appearance
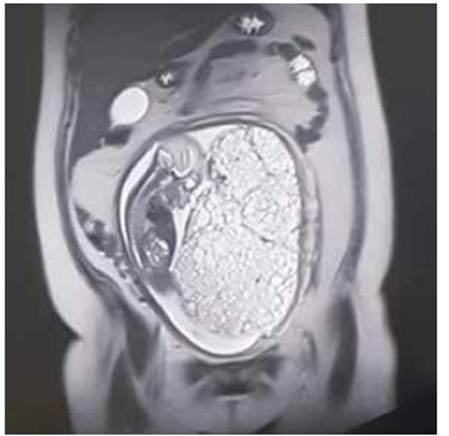
At 21 weeks, amniocentesis was performed to study the fetal karyotype; microarray analysis was not included because it was not available in our institution. The MRI showed a neoformative mass at the inferior placental border, with a diffuse microcystic pattern, measuring 20 x 12 cm along the anteroposterior, crown-rump axis, and compatible with hydatidiform mole (Figure 2).
The patient was evaluated by endocrinology because of a TSH value of 0.01 and a free T4 value of 21.2, compatible with hyperthyroidism, which was attributed to the described tumor. Expectant management was prescribed. However, one week later, thyroxine levels increased (TSH=0.005 and T4L=25.2) and symptoms of hyperthyroidism appeared, so the patient was prescribed thiamazole 5 mg daily.
At 23 weeks, an ultrasound-guided core needle biopsy of the mass was obtained in the operating room, without complications. The MRI showed no metastasis in brain, thorax, abdomen nor pelvis.
At 24 weeks, the immunohistochemical analysis of the biopsy showed placental alkaline phosphatase (PLAP) expression in clusters in a monolayer membrane, β-hCG expression in the membrane and cytoplasm, and 20% ki-67 expression; the tumor was negative for melan A. The sample was too small for a hematoxylin and eosin stain, and the immunohistochemical profile did not confirm choriocarcinoma.
At 25 weeks, the karyotype result was 46, XY, normal. An MRI revealed an 11% growth in comparison with the previous control. The patient was started on actinomycin D because the tumor was catalogued as a high-risk trophoblastic neoplasm, according to the FIGO risk scoring system (12 points). Vincristine and cyclofosfamide were added at week 27, and the patient only took them for one week; they were suspended due to moderate abdominal and digestive pain. The β-hCG level during this time was 1 034 120 mUI/ml.
At 29 weeks, the fetus weighed 1 013 g (p=3.7%), and the middle cerebral artery pulsatility index was under the 5th percentile, compatible with stage 1 intrauterine growth restriction. The hCG-β value was 160 106 mUI/ml. Due to premature rupture of membranes, the patient was programmed for a C-section and hysterectomy, obtaining a male newborn weighing 961 g, Apgar 5 and 7.
The patient had a favorable progress. Thyroid hormones normalized, so thiamazole was suspended. At day 14, the patient was discharged with a hCG-β value of 627, for outpatient follow-up. At the time of this report, the patient remains asymptomatic, and has tested negative for hCG-β in every monthly control. On the other hand, the newborn spent 52 days in the intensive care unit and was afterwards discharged for outpatient follow-up, where he continues at the time of this communication, in the services of ophthalmology and physical therapy.
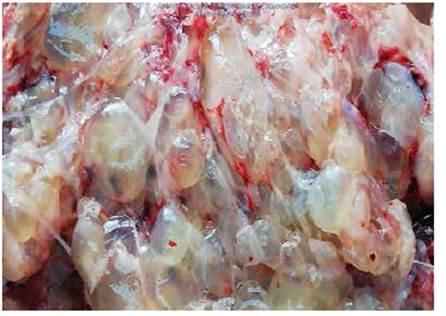
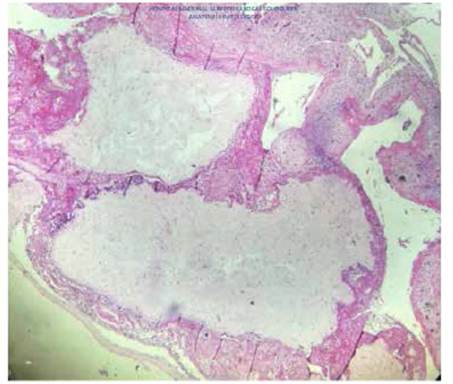
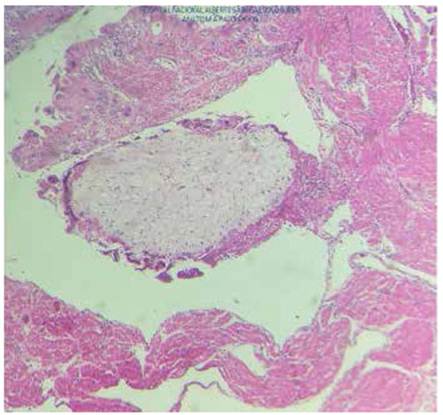
According to the pathology report, the uterus measured 26 cm and contained a normal 17 cm placenta with umbilical cord and chorioamniotic membranes with a faint green tint. Towards the right side, a 23 cm tumor was found filling the uterine cavity and occluding the internal os (Figure 3), composed of 0.4 to 2 cm vesicles containing mucus, similar to a bunch of grapes, with hemorrhagic and necrotic areas (Figure 4). The microscopic examination identified avascular, edematous chorionic villi with mucoid stroma, karyorrhexis, cistern formation, concentric trophoblastic hyperplasia and foci of fibrinoid necrosis and polymorphonuclear neutrophils. At the implantation site, there was high proliferation and mild atypia of the syncytiotropho blast (Figure 5). Slides with a relatively thinner myometrium revealed villi invading up to 2 mm, concordant with invasive mole (Figure 6).
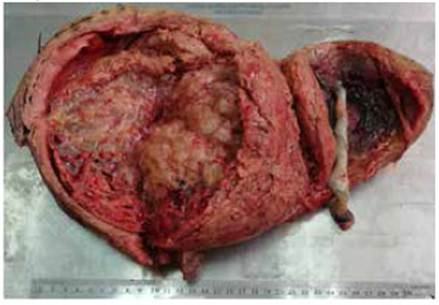
Figure 3 Uterus with a normal, dark placenta and umbilical cord; the adjacent tumor occludes the cervical os.
Discussion
According to our literature search, this is the first reported case of an invasive mole coexisting with a normal, living fetus, with good neonatal outcome.
These cases are a clinical dilemma regarding management. For example, due to fear of maternal complications or trophoblastic neoplasia, logic would mandate immediate intervention.
Nevertheless, because spontaneous regression is possible5, as well as a stationary behavior or a favorable response to chemotherapy, one can opt for expectant management, not without risk, especially in a highly desired pregnancy.
Although the exact cause remains unknown, an association between gestational trophoblastic disease and assisted reproductive treatment has been observed. It has been hypothesized that, if ovulation is induced in more than one egg, there could be a higher risk of obtaining an empty one, which would be susceptible to degenerate into a molar pregnancy6.
Diagnosing gestational trophoblastic disease is hard per se; combined with a concurring pregnancy, it is even more challenging because we must differentiate it, mainly, from partial hydatidiform mole and from placental mesenchymal dysplasia (PMD)5. Precise diagnosis is important, given that prognosis, fetal viability and treatment vary.
Ruling out a partial molar pregnancy is crucial because, in these cases, fetuses are almost always triploid and termination of the pregnancy is indicated. Karyotype analysis by amniocentesis is required7 because almost 92% of triploid fetuses present structural abnormalities6. In our case, the karyotype was normal and the fetus had no malformations.
Placental mesenchymal dysplasia (PMD) is a rare placental vascular anomaly characterized by placentomegaly and vesicles. It affects 0.02% of all pregnancies and is associated to Beckwith-Wiedemann syndrome. Distinguishing this disease from a molar pregnancy by ultrasound alone is hard. The most important factor in the differential diagnosis is the location of the abnormal mass: while a mole lies outside the gestational sac, PMD is located inside. MRI can help in these cases2,8.
While there have been reports of ultrasound diagnosis in the first trimester, it is more frequently diagnosed in the second trimester, in 68 to 92% of the cases6. The snowstorm sign, which appears outside the gestational sac in the second trimester, is highly suggestive. In some series, only 43 to 68% of patients were correctly diagnosed by ultrasound4.
Fowler9 reported the following values for ultrasound diagnosis before evacuation, in patients with confirmed hydatidiform mole in the United States: sensitivity 44%, specificity 74%, positive predictive value 88% and negative predictive value 23%. The detection rate was better for complete moles (58 to 95%) than partial moles (17 to 29%).
No studies have investigated the diagnostic precision of MRI. However, this exam can determine if the trophoblastic disease is separated from the gestational sac, which leads to a more precise diagnosis of GTD coexisting with a healthy fetus, thus ruling out a partial mole with higher precision than ultrasound4.
Hyperthyroidism is the most frequently associated non-gynecologic disease in these cases. This disease’s higher frequency is linked to the increase in hCG-β values because this hormone’s alpha unit is the same as that of TSH, so it also stimulates TSH receptors and the consequent liberation of thyroxine.
Lin10 obtained a frequency of hyperthyroidism of 14% when only considering the cases that presented both clinical signs and laboratory findings of thyroid hormonal imbalance.
In the case we report, symptoms and hormonal alterations correlated with hCG-β values.
Regarding management and prognosis, in 2017, there were less than 250 cases of complete mole with twin pregnancy documented in the literature, and in less than 60 cases, the pregnancy resulted in life births6. This is explained by a fear of a higher risk of persistent disease or progression to neoplasia. However, Lin10 showed that, although the rate of progression to persistent disease was higher in these cases (42%) than in individual moles (18%), it did not reach statistical significance (p=0.0625). Furthermore, Sebine11, in a long series of 77 cases, found no significant difference between the percentage of patients with a mole and a living fetus who chose to end the pregnancy, and those who did not.
So terminating the pregnancy because of fear to develop a gestational trophoblastic neoplasia (GTN) is not justified; it has been proved that there is no association between the elective interruption of the pregnancy and the rate of GTN.
Similarly, the gestational age at evacuation has no influence in the progression to GTN in single pregnancies11.
On the other hand, while there is great variability in the percentage of live newborns, up to 60% with expectant management10, parents who decide to continue with the pregnancy must accept the risk of possible maternal complications associated to the hydatidiform mole. In the same way, its management requires admission to a high-level hospital with an intensive care unit, a variety of specialists, blood bank and experience with high risk pregnancies.
To obtain a viable product, we had to use high technology, like karyotype analysis, ultrasound, MRI, tumor biopsy, immunohistochemistry and hormone assays, among others.
In conclusion, we report the first case in the literature of an invasive mole with a normal fetus, with neonatal viability, using the technology available in our hospital. Molar pregnancy with a healthy fetus is a rare event and its management is still uncertain. It has not been demonstrated that expectant management increases the risk of progression to trophoblastic neoplasia, nor that early pregnancy termination decreases this risk. However, if it is decided to continue the pregnancy, this will require monitoring in high-level hospitals with availability of several specialties and sufficient technology to manage high risk pregnancies and their complications.
REFERENCES
1. Kurman RJ, Carcangiu ML, Herrington S, Young RH. Gestational trophoblastic disease. En: Kurman RJ, Carcangiu ML, Herrington CS, Young RH (editores). WHO classification of tumours of female genital reproductive organs. 4ta edición. Lyon: International Agency for Research on Cancer. 2014. [ Links ]
2. Seo SY, Cho HJ. Twin pregnancy with complete hydatidiform mole and co-existent live fetus. J Fetal Med. 2016;3:41-4. DOI 10.1007/s40556-015-0070-y [ Links ]
3. Johnson C, Davitrt C, Harrison R, Cruz M. Expectant management of a twin pregnancy with complete hydatidiform mole and coexistent normal Fetus. Case Rep Obstet Gynecol. 2019;2019:8737080. doi.org/10.1155/2019/8737080 [ Links ]
4. Imafuku H, Miyahara Y, Ebina Y, Yamada H. Ultrasound and MRI findings of twin pregnancies with complete hydatidiform mole and coexisting normal fetus: Two case reports. Kobe J Med Sci. 2018;28(64):E1-E5. doi.org/10.24546/81010321 [ Links ]
5. Radhouane A, Imen BA, Khaled N. Twin pregnancy with both complete hydatiform mole and coexistent alive fetus: Case report. Asian Pac J Reprod. 2015;4:331-3. doi: 10.1016/j.apjr.2015.07.013 [ Links ]
6. Giorgione V, Cavoretto P, Cormio G, Valsecchi L, Vimercati A, De Gennaro A, et al. Prenatal diagnosis of twin pregnancies with complete hydatidiform mole and coexistent normal fetus: A series of 13 cases. Gynecol Obstet Invest. 2017;82(4):404-9. doi: 10.1159/000448139 [ Links ]
7. Mohiuddin KM, Ratha C, Satishrao I. Complete hydatidiform mole with a coexistent live fetus in a twin pregnancy. J Med Sci Res. 2017;5:111-4. DOI: 10.17727/JMSR.2017/5-21 [ Links ]
8. Himoto Y, Kido A, Minamiguchi S, Moribata Y, Okumura R, Mogami H., et al. Prenatal differential diagnosis of complete hydatidiform mole with a twin live fetus and placental mesenchymal dysplasia by magnetic resonance imaging. J Obstet Gynaecol Res. 2014;40:1894-900. doi: 10.1111/jog.12441 [ Links ]
9. Fowler DJ, Lindsay I, Seckl MJ, Sebire NJ. Routine pre-evacuation ultrasound diagnosis of hydatidiform mole: experience of more than 1000 cases from a regional referral center. Ultrasound Obstet Gynecol. 2006;27:56-60. doi: 10.1002/uog.2592 [ Links ]
10. Lin LH, Maestá I, Braga A, Sun SY, Fushid K, Francisco RPV, et al. Multiple pregnancies with complete mole and coexisting normal fetus in North and South America: A retrospective multicenter cohort. Gynecol Oncol. 2017 Apr;145(1):88-95. DOI: 10.1016/j.ygyno.2017.01.021 [ Links ]
11. Sebire NJ, Foskett M, Paradinas FJ, Fisher RA, Francis RJ, Short D, Newlands ES, Seckl MJ. Outcome of twin pregnancies with complete hydatidiform mole and healthy co-twin. Lancet. 2002;359:2165-6. DOI: 10.1016/S0140-6736(02)09085-2 [ Links ]
6Citar como: Tipiani Rodríguez O, Solís Sosa C, Valdez Alegría GE, Quenaya Rodríguez RJ, Escalante Jibaja R, Cevallos Pacheco C, Ibarra Lavado O, Bocanegra Becerra YL. Mola invasiva coexistente con feto vivo normal. Reporte de caso. Rev Peru Ginecol Obstet. 2020;66(2):DOI: https://doi.org/10.31403/rpgo.v66i2253
Received: February 07, 2020; Accepted: April 04, 2020











 texto en
texto en