Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.2 Lima Apr-Jun 2020
http://dx.doi.org/10.31403/rpgo.v66i2256
Case report
Gliomatosis peritonei in mature ovarian teratoma
1 Obstetrics and Gynecology Service, Central Hospital "Dr. Urquinaona", Maracaibo, Zulia State, Venezuela
Ovarian teratomas are tumors derived from the three germinal layers. Gliomatosis peritonei is a rare condition characterized by glial tissue implants in peritoneum, commonly associated with immature ovarian teratoma, but not with mature teratomas. Definitive diagnosis is based on histopathological examination. Currently, there are no guidelines for its follow-up. Prognosis is favorable; however, implants may present fibrotic regression or exceptionally degenerate into glioblastoma. Treatment should be similar to that of metastatic ovarian carcinoma if immature glial tissue or other teratomatous components are found in peritoneum or omentum. Long-term follow-up is necessary in patients with residual peritoneal disease due to risk of recurrence and malignant transformation. We present a case of gliomatosis peritonei associated to mature ovarian teratoma.
Key words: Ovarian neoplasms; Teratoma; mature; Ovary
Introduction
Gliomatosis peritonei is a rare entity characterized by the presence of benign glial implants in peritoneum, omentum and lymph nodes. It is generally associated with immature ovarian teratomas and rarely with mature teratomas1. Because of its low frequency, there are no protocols for its follow-up, and prognosis has not been established. Some reports link it to pregnancy and ventriculoperitoneal shunts for hydrocephalus2,3. We present a case of gliomatosis peritonei associated with a mature ovarian teratoma.
Case report
A 21-year-old patient, gravida 3, para 2, abortus 1, consulted for intermittent, nonradiating pain of moderate intensity in the right iliac fossa for 7 days, associated to abdominal bloating, increased body temperature and loss of appetite. She denied any urinary alterations, changes in bowel movements, weight loss, chronic or endocrine diseases, cancer, smoking, using prescription or illicit drugs, and had no contributing family history. She had smoked approximately 10 cigarettes a day for the last two years. Regarding her gynecological history, she experienced menarche at age 14, and had irregular periods every 2 to 3 months, lasting 6 to 7 days; her first sexual intercourse was at age 16. Her last period had happened 13 days before her symptoms started.
At physical examination, the patient was conscious and oriented. Heart rate, blood pressure and respiratory rate were normal. Abdomen was soft and depressible; bowel sounds were present. We palpated a soft tumor located 2 cm above the navel, slightly movable, painful on deep palpation. Pelvic examination revealed pain on movement of the cervix. Cervix was closed, without evidence of bleeding or vaginal discharge. We could not palpate the tumor with this method. Upon rectal exam, anal sphincter tone and rectal cavity were normal, and there was a palpable tumor occupying part of the pouch of Douglas.
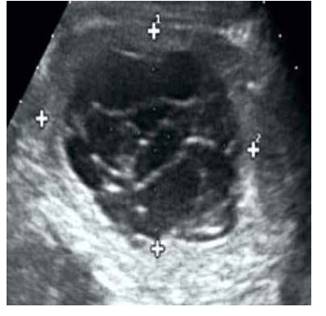
Transvaginal ultrasound showed a homogenous uterus of smooth contours measuring 8 x 3 x 3 cm, with hyperechoic, thickened endometrium (11 mm) and intact subendometrial halo. It also revealed a hyperechoic, heterogeneous abdominopelvic tumor measuring 20 x 19 x 11 cm, probably originating in the right ovary, presenting multiple cysts of variable size and a solid area of 4 x 1 cm. Its walls were thick and measured 4 mm, and it had multiple septations 3 mm wide, with papillary outgrowth and projections in the internal portion; we assigned it a Sassone score of 12 (Figure 1). The left ovary measured 3 x 2 x 2 cm. There was a small amount of free intraperitoneal fluid, and minimal right pyelectasis. Ultrasound showed no alterations in the other organs. Blood tests, liver and renal function tests, electrolytes and coagulation profile were within normal values. Beta-hCG was negative.
Concentrations of tumor markers CA-125 (165 UI/mL, reference value (RV) under 35 UI/mL) and alpha-fetoprotein (40,5 ng/mL, RV under 10 ng/mL) were elevated. Given these findings, we considered the possibility of a malignant ovarian tumor.
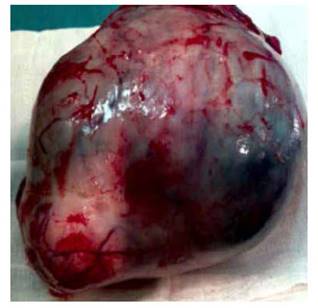
In the exploratory laparotomy, we found approximately 200 mL of free intraperitoneal fluid, which we sampled for cytology. The right ovary presented a multiloculated cystic tumor measuring 20 x 17 x 13 cm, with its capsule intact (Figure 2). The fallopian tube was elongated and strongly adhered to the tumor. The omentum had a spherical tumor of approximately 2 cm in diameter, with multiple implants in appendix, intestines and colon. There was no seeding in diaphragm, liver, gallbladder, spleen and kidneys. We resected the lesion in omentum, took a sample of the peritoneal implants and of several portions of the ovary and sent them for frozen section biopsy, which was informed as mature cystic ovarian teratoma and gliomatosis peritonei. We performed right oophorosalpingectomy, partial omentectomy, comprehensive surgical staging, appendectomy, biopsy of some of the peritoneal implants, bilateral pelvic lymphadenectomy, and biopsy of some paraaortic lymph nodes. The patient tolerated the procedure well and the surgery ended without complications.
Microscopic evaluation showed mature tissue derived from the three germinal layers with ectodermal predominance and an organized pattern, with skin next to skin appendages, columnar epithelium, fat, bone, cartilage and neural differentiation. Implants in omentum and peritoneum were gray focal areas with multiple nodes containing fibroconnective and adipose tissue with mesothelial lining and concentric aggregates of mature glial tissue, associated to peripheral nonspecific mixed inflammatory reaction (Figure 3). Immunostaining was positive for glial fibrillary acidic protein (GFAP) and S100 protein. There was no immature tissue nor signs of malignancy. The rest of tissue samples showed no neoplastic growth. Final diagnosis was mature cystic teratoma of the right ovary associated with gliomatosis peritonei.
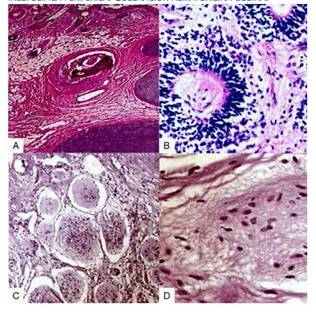
Figure 3 a) ovarian tissue with squamous and columnar epithelium, pilous follicles, fat and cartilage. b) section from the ovarian teratoma, with skin and skin appendages. c) peritoneal nodes, with fibroconnective and adipose tissue, and mesothelial lining with mature glial tissue. d) glial tissue with nonspecific peripheral inflammatory response. hematoxylin-eosin staining.
The patient had no post-operative complications and was discharged 4 days later. We reevaluated her one week after discharge; afterwards, she did not return for the follow-up consultation.
Discussion
Ovarian teratomas are tumors composed of tissue derived from the three embryonic germ layers (endoderm, mesoderm and ectoderm), present in varying quantities within the tumor. Gliomatosis peritonei is a condition where glial tissue implants appear in peritoneum; its peak incidence occurs during the second decade of life4. The association of gliomatosis peritonei with immature ovarian teratoma is rather common, unlike its association with mature teratomas5. Given its low frequency - there are only circa 100 reports -, we do not know its real incidence2.
Several theories propose a pathogenesis for this condition and possible factors responsible for its development in association with ovarian teratomas. Some authors suggest that glial implants come directly from the teratoma, possibly by rupture. This mechanism is based on the presence of implants in omentum close to capsule defects. The theory of dissemination by angiolymphatic vessels builds on the findings of mature glial tissue in paraaortic and pelvic lymph nodes in patients with immature ovarian teratoma6. It has also been proposed that the implants do not come from the teratoma, but from pluripotent stem cells in peritoneum, due to genetic analyses evidencing differences between glial implants and teratomas. This phenomenon may happen because of pluripotent Müller cells undergoing glial metaplasia in the peritoneal surface or subcelomic mesenchyma in response to factors secreted by the teratoma, which may induce specific differentiation pathways. The occurrence of gliomatosis peritonei in patients with ventriculoperitoneal shunt, which would allow cerebrospinal fluid to transport glial tissue, supports this theory. Nevertheless, the specific mechanisms by which peritoneal cells undergo glial transformation remain unknown7.
Alpha-fetoprotein is a serum marker used for the diagnosis and follow-up of germ cell tumors. Its use in the follow-up of patients with ovarian teratoma may be hindered by its low sensitivity and specificity, although it could help rule out metastasis of immature ovarian teratomas8. Concentrations may remain within normal values in cases of gliomatosis peritonei, even if they were elevated with the original teratoma. They may also appear normal in recurrent tumors containing immature elements7.
Glial peritoneal implants are small and may be in parietal or visceral peritoneum. Pelvic structures can be evaluated by transvaginal ultrasound. However, implants are usually very small (smaller than 3 mm), so they may not be detected. They can also be hidden by intestinal gas, or outside the visualization field9. CT SCAN and MRI may provide a better detection, although their etiology may be confused with carcinomatosis or peritoneal tuberculosis(10). MRI may only show big implants. T2 weighted images offer the best resolution because implants have a moderately high signal intensity. PET SCAN may also be useful for diagnosing and monitoring these lesions9.
The definitive diagnosis of gliomatosis peritonei is based on histopathological findings, often in sections stained with hematoxylin and eosin. Differentiating this condition from epithelial low-grade ovarian tumors may be difficult. Peritoneal implants appear as small white nodules, may contain mature or immature glial tissue, and are generally more differentiated than the primary tumor11. These implants may transform into fibroblasts and afterwards disappear, persist without morphologic changes, or undergo malignant transformation4. They are generally positive for markers like GFAP, S-100 protein and neuron-specific enolase. Immunostaining with GFAP determines if the glial tissue is mature and well differentiated, thus confirming diagnosis12.
Due to this condition’s low frequency, there is no single accepted algorithm for treatment, length and type of follow-up. Surgery generally consists in the resection of the tumor and implants by exploratory laparotomy, which allows exploring the abdominal cavity and taking samples of peritoneal fluid for cytological study and biopsies of peritoneal implants. If associated with mature ovarian teratoma, surgical extraction of the primary tumor may be enough1. In cases associated with immature teratoma, it is necessary to perform a comprehensive surgical staging and to take samples of the highest possible amount of implants, because management and prognosis depend on the presence of immature glial elements in them. If they are present, treatment has to be like that of metastatic ovarian carcinoma13.
Options of chemotherapy include the combination bleomycin, etoposide and cisplatin or vincristine, adriamycin, cyclophosphamide. The latter is usually reserved for recurrent cases(2,14). If there are immature glial tissue or other teratomatous components in peritoneum or omentum, treatment has to be similar to that of metastatic ovarian carcinoma. The presence of glial tissue in lymph nodes is rare, so adjuvant chemotherapy is not necessary if this glial tissue is mature2.
Gliomatosis peritonei in itself has low malignant potential. Its prognosis relates to the type of the primary ovarian teratoma. Survival rate at 5 years varies between 30 and 82%, depending on histologic findings and surgical staging. Good prognosis is also associated with implants composed exclusively of mature glial tissue, although recurrence is more frequent and disease-free survival is shorter. Implants may remain stable, undergo fibrous regression, or transform into glioblastoma11. Long-term follow-up is necessary to rule out malignant transformation, given that some cases progress to malignancy up to 7 years after surgery, especially when associated to immature ovarian teratoma or in cases without comprehensive surgical staging, histologic evaluation of implants and treatment with chemotherapy5,13. Until now, there have been no reports of malignant transformation of gliomatosis peritonei associated to mature cystic teratoma. Patients with immature ovarian teratoma and glial implants have a better prognosis if peritoneal surface, omentum and diaphragmatic surfaces have implants exclusively or almost exclusively composed of mature glial tissue9,15.
In conclusion, gliomatosis peritonei consists of mature glial tissue implants in peritoneal surface. It is often associated with immature ovarian teratomas, not with mature teratomas. Despite the inactivity of residual peritoneal disease, it requires long-term follow-up. Prognosis is generally favorable.
REFERENCES
1. Bentivegna E, Gonthier C, Uzan C, Genestie C, Duvillard P, Morice P, et al. Gliomatosis peritonei: a particular entity with specific outcomes within the growing teratoma syndrome. Int J Gynecol Cancer. 2015;25(2):244-9. doi: 10.1097/IGC.0000000000000345 [ Links ]
2. Wang D, Jia CW, Feng RE, Shi HH, Sun J. Gliomatosis peritonei: a series of eight cases and review of the literature. J Ovarian Res. 2016;9(1):45. doi: 10.1186/s13048-016-0256-5 [ Links ]
3. Wu PS, Lai CR. Ovarian immature teratoma with gliomatosis peritonei and pleural glial implant: a case report. Int J Surg Pathol. 2015;23(4):336-8. doi: 10.1177/1066896915570361 [ Links ]
4. Müller AM, Söndgen D, Strunz R, Müller KM. Gliomatosis peritonei: a report of two cases and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2002;100(2):213-22. [ Links ]
5. Badru F, Saxena S, Munoz-Abraham AS, Guzman MA, Bansal S, Chatoorgoon K. Peritoneal nodules in a pediatric patient with benign teratoma. A case report and review of literature. J Pediatr Adolesc Gynecol. 2018;31(6):632-636. doi: 10.1016/j.jpag.2018.07.001 [ Links ]
6. Kwan MY, Kalle W, Lau GT, Chan JK. Is gliomatosis peritonei derived from the associated ovarian teratoma? Hum Pathol. 2004;35(6):685-8. [ Links ]
7. Ferguson AW, Katabuchi H, Ronnett BM, Cho KR. Glial implants in gliomatosis peritonei arise from normal tissue, not from the associated teratoma. Am J Pathol. 2001;159(1):51-5. [ Links ]
8. Liang L, Olar A, Niu N, Jiang Y, Cheng W, Bian XW, et al. Primary glial and neuronal tumors of the ovary or peritoneum: A clinicopathologic study of 11 cases. Am J Surg Pathol. 2016;40(6):847-56. doi: 10.1097/PAS.0000000000000635 [ Links ]
9. Lavoie JM, Lacroix-Poisson F, Hoang LN, Wilson DC, Seckl MJ, Tinker AV. 18F FDG positron-emission tomography findings of gliomatosis peritonei: A case report and review of the literature. Gynecol Oncol Rep. 2017 Mar 27;20:105-7. doi: 10.1016/j.gore.2017.03.018 [ Links ]
10. Khan J, McClennan BL, Qureshi S, Martell M, Iyer A, Bokhari SJ. Meigs syndrome and gliomatosis peritonei: a case report and review of literature. Gynecol Oncol. 2005;98(2):313-7. [ Links ]
11. Chan JK, Gardner AB, Chan JE, Guan A, Alshak M, Kapp DS. The influence of age and other prognostic factors associated with survival of ovarian immature teratoma A study of 1307 patients. Gynecol Oncol. 2016;142(3):446-51. doi: 10.1016/j.ygyno.2016.07.001 [ Links ]
12. Gu S, Wu YM, Hong L, Zhang ZD, Yin MZ. Glial fibrillary acidic protein expression is an indicator of teratoma maturation in children. World J Pediatr. 2011;7(3):262-5. doi: 10.1007/s12519-011-0258-8 [ Links ]
13. Liang L, Zhang Y, Malpica A, Ramalingam P, Euscher ED, Fuller GN, et al. Gliomatosis peritonei: a clinicopathologic and immunohistochemical study of 21 cases. Mod Pathol. 2015;28(12):1613-20. doi: 10.1038/modpathol.2015.116 [ Links ]
14. Kang H, Kim TJ, Kim WY, Choi CH, Lee JW, Kim BG, et al. Outcome and reproductive function after cumulative high-dose combination chemotherapy with bleomycin, etoposide and cisplatin (BEP) for patients with ovarian endodermal sinus tumor. Gynecol Oncol. 2008;111(1):106-10. doi: 10.1016/j.ygyno.2008.05.033 [ Links ]
15. Webman R, Talishinskiy T, Raetz E, Lala S, Tomita S. Spontaneous regression of thoracic and extraperitoneal glial implants in child with gliomatosis peritonei after resection of ovarian teratoma. J Pediatr Hematol Oncol. 2015;37(3):230-1. doi: 10.1097/MPH.0000000000000230 [ Links ]
Received: August 13, 2019; Accepted: October 09, 2019











 text in
text in