Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.2 Lima Apr-Jun 2020
http://dx.doi.org/10.31403/rpgo.v66i2258
Letters to the Editors
Interpretation of tests for COVID-19 in the pregnant woman
1Editor, The Peruvian Journal of Gynecology and Obstetrics; Expert Extraordinary Professor Faculty of Medicine, Universidad Nacional Mayor de San Marcos, Lima-Peru;Past President Sociedad Peruana de Obstetricia y Ginecología; Fellow American College of Obstetricians and Gynecology.
2Member Editorial Committee RPGO; Past President Fundación Instituto Hipólito Unanue, Lima-Perú; Fellow and Vice Chair Peru American College of Obstetricians and Gynecologists
3Member Editorial Committee RPGO; Past President Sociedad Peruana de Obstetricia y Ginecología: Fellow and Chair Peru American College of Obstetricians and Gynecologists
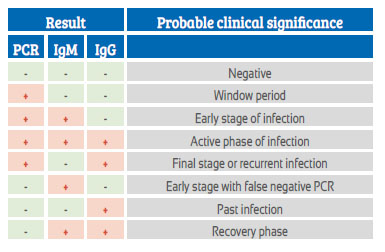
In the publication of Lorena Campodónico Olcese et al. ‘Management of eutocic delivery in a patient with COVID-19 in Lima, Peru'1, we read in the case report the following comment on the test results for for COVID-19: "... since there is a percentage of asymptomatic carriers, ... universal screening with both tests is performed at the clinic on all patients ad- mitted for vaginal delivery or C-section. Requesting both tests benefits the diagnosis of patients in whom the molecular RT-PCR test may be un- noticeable by low viral load, which happens about two weeks after the onset of symptoms. On the other hand, IgM and IgG antibodies reach or start their peaks in the second or third week of symptoms onset and decline around the fifth to seventh week." "In the labor room, a rapid blood test (CELLEX SARS-CoV-2 IgG/IgM Rapid Test) and a nasopharyn- geal swab molecular test (RT-PCR SARS-CoV-2) for COVID-19 were taken. The rapid test result was IgM (+) IgG (-). A week after delivery, the results were IgM (+) IgG (-) and negative molecular test, and a chest CT was re- ported as normal."
The consideration of this case as positive to COVID-19 despite negative molecular evidence has drawn attention. In the literature it is pointed out that RT-PCR results suggest that viral loads may be detected soon after the onset of the disease, even in minimally symptomatic people 2. One or more negative results do not rule out the possibility of infection3. And a percentage of negative tests may be due to inappropriate sampling4.
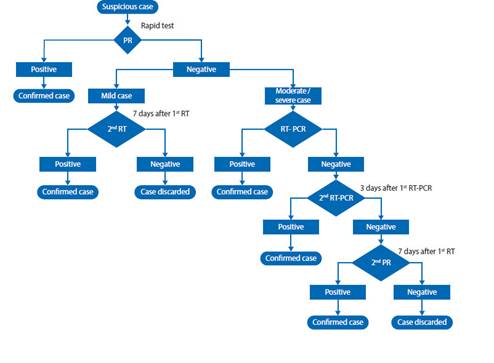
These concepts are supported by various publications by laboratory experts such as Sethraman (Figure 1) 5 and Pineda (Figure 2) 6, and the Peruvian Ministry of Health Health Directive flowchart (Figure 3) 7.
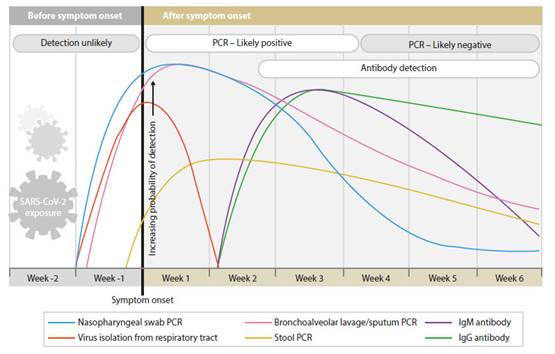
Figure 1 beginning and variation of the positivity of the tests for the diagnosis of sars-cov-2, in relation to the onset of symptoms5.
On the other hand, in the World Association of Perinatal Medicine's 9 May 2020 webinar on COVID-19 in pregnancy, Gabriele Saccone of Italy reported that two consecutive negative qRT-PCR tests are considered to rule out that the pregnant woman has COVID-198.
As curves and opinions currently diverge in publications, it is important to emphasize, especially in times of the rising epidemic curve, that the approach should be to consider everyone positive or infected until proven otherwise.
Symptoms, anamnesis, adequate interpretation of the results of diagnostic tests (molecular or serological), the time of the disease when the tests are taken, the correct sampling, among others, should be factors to consider by the attending physician to reach an adequate diagnosis, management and treatment.
The error of antibody tests is a current concern around the world. Depending on the test, it may have a sensitivity of 100% and a specificity of 99.8%, so only 0.2% of people without antibodies are considered as if they had the virus. Pre-exposure to other coronaviruses does not affect results9. Therefore, the criterion that a negative test does not negate and a positive affirms it, must be valid, especially in these times of epidemic.
One of the authors (AGC) of this letter considers that, in Campodónico’s case report, 4 weeks symptomatic (although it was just cough), perhaps the molecular test taken at the beginning was no longer diagnostic (if it had been COVID-19, viral load would have already dropped) and whether the diagnosis with rapid tests was more useful. However, it turned out IgM was positive which AGC believes is not specific and not IgG, which at that time it should be positive or a week later. AGC’s opinion is that it was not a COVID-19 patient, but he considers this case report is valid because it explains the ideal protocol in an obstetric setting.
In the U.S. National Institute of Health first quick review series publication, based on two studies developed in China published in 2020, the combined IgG and IgM antibody screening test showed good sensitivity (87-88%) and specificity (90-100%) for diagnosis for SARS-CoV-2 compared to RT-PCR. Diagnosis accuracy was higher when IgG and IgM were detected simultaneously. The utility of the positive and negative predictive value calculated by the studies (96-100% and 72-81%, respectively) is limited, considering that at this time the disease prevalence in our country is significantly lower than reported in other studies (>60%), as we are at an early stage of the epidemic10.
Another publication notes that test results can be false positive, false negative, or ambiguous, causing great confusion for diagnosis and follow-up11. About 60 different tests for RNA or antibodies are available in the U.S. after authorization by the U.S. Food and Drug Administration for use during the emergency.
On the other hand, variation can be due to virus variants or genetic variants of people and their response to the virus.
As curves diverge in publications, we suggest that in any asymptomatic or symptomatic pregnant woman in labor, a good anamnesis should be performed for COVID-19 symptoms and SARSCoV-2 TR-PCR molecular tests and IgM and IgG antibodies be requested. A negative test does not deny and a positive test affirms the probability of occurrence of the disease, and the pregnant woman should be managed as such.
For the final diagnosis, consideration will be given to the clinical evolution of the puerperal woman and her baby and the results of the new tests in both, with special care when taking the sample for molecular testing to be analyzed in quality laboratories. With more experience, this controversy will be clarified.
REFERENCES
1. Campodónico Olcese L, Paredes Salas JR, Campodónico Olcese D, Chang Vargas C, Acuña Barrueto L, Marchena Arias J. Atención de parto eutócico en gestante con COVID-19 en Lima - Perú. Rev Peru Ginecol Obstet: 2020;66(2): DOI: https://doi.org/10.31403/rpgo.v66i2251 [ Links ]
2. Chow EJ, Schwartz NG, Tobolowsky FA, Zacks RLT, Huntinhton-Frazier M, Reddy SC, Rao AK. Symptom screening at illness onset of health care personnel with SARS-CoV-2 infection in King County, Washington. JAMA. 2020 Apr 17;e206637. doi: 10.1001/jama.2020.6637. Online ahead of print. [ Links ]
3. Saxena SK. Coronavirus Disease 2019 (COVI-19). Epidemiology, Pahthogenesis, Diagnosis and Therapeutics. En: Saxena SK, Editor. Medical Virology: form pathogenesis to disease control. Springer Nature Singapore Pte Ltd. 2020. https://doi-org/10.1007/978-981-15-4814-7 [ Links ]
4. Pacheco J. El enigma del coronavirus, la gestante y su niño. Lo que el ginecobstetra está conociendo. Rev Peru Ginecol Obstet. 2020;66(2). En prensa. DOI: https://doi.org/10.31403/rpgo.v66i2247 [ Links ]
5. Sethraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. Published online: May 6, 2020. doi:10.1001/jama.2020.8259 [ Links ]
6. Pineda Tenor D, Rodríguez Borja E, Gascón Luna F, Pacheco Delgado M, Lorenzo Lozano MC, Prada de Medio E; Asociación Española de Biopatología Médica. CPVID-19. Perspectiva desde el laboratorio clínico. Abril 2020. https://www.semae.es/wp-content/uploads/ASOCIACION-ESPA%C3%91OLA-DE-BIOPATOLOGIA-MEDICA-Informe-Co-Vid19-Abril-2020.pdf [ Links ]
7. Ministerio de Salud. Centro de Epidemiología, Prevención y Control de Enfermedades. Alerta Epidemiológica Código: AE-015-2020. Alerta Epidemiológica ante la transmisión de COVID-19 en el Perú. Abril 2020. https://www.dge.gob.pe/portal/docs/alertas/2020/AE015.pdf [ Links ]
8. World Association of Perinatology. World school of Perinatal Medicine. COVID-19 in pregnancy. Webinar 09 May 2020. https://wspm.perinatal.or.tr [ Links ]
9. Lowewy MA. COVID-19. Resumen semanal (1-7 mayo 2020). Medscape. 8 de mayo de 2020. https://espanol.medscape.com/verarticulo/5905399?src=mkm_latmkt_200510_mscmrk_mdsms_excnws_nl&uac=134705ST&impID=2374976&faf=1 [ Links ]
10. Instituto Nacional de Salud (Perú). Precisión diagnóstica de pruebas rápidas de detección de anticuerpos para SARSCoV-2. Elaborado por Adolfo Aramburu. Lima: Unidad de Análisis y Generación de Evidencias en Salud Pública. Instituto Nacional de Salud, Marzo de 2020. Serie Revisiones Rápidas Nº 01-2020. https://web.ins.gob.pe/sites/default/files/Archivos/authenticated%2C%20administrator%2C%20editor/publicaciones/2020-04-15/RR%2001%20Pruebas%20rapidas%20SARS-CoV-2%20-%20Serolog%C3%ADa_V.02_final.pdf [ Links ]
11. Allen J. 'Like a science experiment': A New York family learns the limits of coronavirus tests. May 7, 2020. https://news.yahoo.com/science-experiment-york-family-lear-ns-110325762.html?.tsrc=daily_mail&uh_test=1_04 [ Links ]
Received: May 07, 2020; Accepted: May 12, 2020











 text in
text in