Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.3 Lima Jul-Sep 2020
http://dx.doi.org/10.31403/rpgo.v66i2259
Original Articles
SARS-COVID-19 antibodies in pregnant women at a level III hospital in Peru
1Instituto Nacional Materno Perinatal. (National Maternal Perinatal Institute) Lima, Perú
2Universidad Nacional Mayor de San Marcos, School of Human Medicine. Lima, Perú
Introduction: COVID-19 disease spreads rapidly. Seroprevalence in pregnant women entering for hospitalization and clinical characteristics in this type of population in Peru is not known. Objective: To determine the prevalence and clinical-epidemiological characteristics of pregnant women with anti-SARS-CoV-2 antibodies at a level III hospital in Peru. Methods: Observational and cross-sectional study performed at the National Maternal Perinatal Institute of Peru. Pregnant women admitted for hospitalization were screened for COVID-19 infection. Results of anti-SARSCoV-2 serological tests and information on maternal and perinatal characteristics were obtained. Data analysis was performed using descriptive statistics and 95% confidence intervals. Results: In 2 419 pregnant women screened we identified a prevalence of 7.0% of anti-SARS-CoV-2 antibodies (95% IC: 6.1% to 8.1%), including IgM in 10% (95% IC: 6.1% to 15.8%), IgM / IgG in 78.8% (95% IC: 71.8% to 84.6%), IgG in 11.2% (95% IC: 7.0% to 17.1%). 89.4% of the seropositive pregnant women were asymptomatic. Most frequent complications were premature rupture of membranes (11.8%) and preeclampsia (6.5%). No association was found between clinical and epidemiologic characteristics and type of serological response to SARS-CoV-2 (p > 0.05). Conclusions: Pregnant women had prevalence of anti-SARS-CoV-2 antibodies of 7.0% on admission to the hospital; most of them were asymptomatic. There was no association between clinical-epidemiological characteristics analyzed and type of anti-SARS-CoV-2 antibody response.
Key words: Pandemic coronavirus infections; 2019-nCoV; prevalence; Pregnancy; Pregnancy complications; Peru
Introduction
COVID-19 disease spreads rapidly in the world's population, from asymptomatic forms to severe cases1, resulting in an overall mortality rate of 5.98%, which varies by region2. In the context of the health emergency in Peru and the world, it is important to know vulnerable groups, with pregnant women being a particularly susceptible group3,4. In previous coronavirus studies, adverse maternal results5 have been reported; however, it is not yet clear what effect the new SARS-CoV-2 coronavirus can have on pregnancy and the fetus6,7. Most pregnant women are asymptomatic, but when they have symptoms, the most common are fever, cough and dyspnea, similar to non-pregnant patients3.
The disease clinic is divided into three periods: viremia phase, acute phase and recovery phase. Diagnostic tests, both molecular RT-PCR and serological detection of immunoglobulins, may be applied, according to specific indications8. Although the RTPCR molecular test is recommended for diagnosis, it is limited in scenarios where resources are limited for development and implementation. Meanwhile, serological testing contributes to clinical diagnosis and epidemiological surveillance9,10. Serological tests may be useful for the identification of asymptomatic infections, and contribute to clinical diagnosis in patients who have not had molecular tests11,12. Moreover, in a context where the infection curve is increasing and resources are limited10.
The objective of this study was to determine the prevalence and clinical-epidemiological characteristics of pregnant women with anti-SARSCoV-2 antibodies attended at the National Perinatal Maternal Institute.
Methods
This an observational, transversal, descriptive and associative study accomplished at the National Maternal-Perinatal Institute (as of now, INMP), a third level of attention establishment of the Health Ministry of Peru, category III-2 and a national reference center. Due to the declaration of state of national sanitary emergency caused by the COVID-19 pandemic, the establishment is now attending only emergencies and provides SARS-CoV-2 antibodies serological testing to every pregnant woman fulfilling criteria for hospitalization, as determined by the medical specialist due to labor or pregnancy morbidity.
All pregnant women hospitalized between April 15 and May 15, 2020 participated in the study if they fulfilled the following inclusion criteria: a) hospital admission indicated by the specialist; b) SARS-CoV-2 antibodies serological on admittance; c) having the SARS-CoV-2 antibodies serological test results. Exclusion criteria were: a) under-registration of the study variables in the medical record; b) not having the results of the serological tests requested for admission.
The serological test used in the institution is the One Step Test Kit Covid-19, a rapid test certified and authorized by the European Community, which is required for consumable goods produced in Asia and commercialized in the Schengen territory. The test required samples of serum/plasma and immunochromatography to simultaneously detect IgM and IgG immunoglobulins; test efficiency is described in the insert specifications. Sensitivity for IgM immunoglobulin detection is 94% and specificity 100% with total coincidence of 97.1%. Sensitivity for IgG immunoglobulin detection is 94% and specificity 100% with a total coincidence of 97.1%. Overall sensitivity for the detection of both immunoglobulins is 97.6%, joint specificity 100% and total coincidence 98.8%.
The procedure was conducted by laboratory personnel trained on the application of said test, following the indications on the insert. The interpretation of results was according to the presence and/or lack of IgM or IgG, according to the test insert indications. For negative IgM / negative IgG, there is no infection, or it is in a very precocious stage. For positive IgM / negative IgG, acute infection in early stage. For positive IgM / positive IgG, acute infection in more evolved stage. For negative IgG / positive IgG, infection in stage of resolution.
Variables considered in the study were: maternal age indicated in years previous to the pregnancy adolescent (younger than 19 years), adult (from 19 to 34 years) and advanced maternal age (35 years old or more)-; trimester of pregnancy considered by date of last menstruation or by ultrasound in the first trimester; parity, indicated as nulliparous when there was no previous labor, primiparous when there was one previous labor and multiparous when there were two or more labors; results of the rapid test, either for IgM, IgG, or both; prenatal controls, considering 6 or more an adequate number of controls; education level: primary (middle school), secondary (high school) or superior; civil status: married, cohabiting, divorced, single or widowed; respiratory symptoms present or absent (considering cough, sore throat, headaches, fever, chills and/ or nasal congestion) at hospital admission; type of pregnancy complication (abortion, threatened preterm birth, ectopic pregnancy, bleeding in the second half of pregnancy, hyperemesis gravidarum, placental insufficiency, preeclampsia, premature rupture of membranes, dysfunctional labor, pneumonia, urinary tract infection); type of labor: C-section or vaginal delivery.
Results of the rapid tests conducted on each pregnant woman were obtained from the standardized cards for serological tests of the Epidemiology and Environmental Health Office of the INMP, and recollection of maternal information from every pregnant woman’s clinical record. Measurement biases when collecting information in retrospective studies were reduced by comparing the information on each standardized serological test card, performed by one investigator, with the respective information in the clinical chart from patient's admission to discharge with the definitive diagnoses, performed by a different investigator.
Bivariate descriptive statistics using contingency tables with absolute and relative frequency summaries were used to describe the results. Inferential analysis consisted of the estimation of 95% confidence intervals. Cross-association measurements between variables were performed using Fisher's exact test, with a significance level of 0.05. Processing of data and graph used the R statistical software version 4.0.
Regarding ethical considerations, the research had approval of the Research Ethics Committee of the National Maternal Perinatal Institute, and from the corresponding institutional permit. The data were collected retrospectively and safe guarding the participants identity.
Results
During the study period, 2 427 pregnant women admitted to the hospital were screened. Eight patients were excluded because they did not have a medical history at the time of the study. Finally, the study was conducted with 2 419 patients who met the selection criteria. Of these, 170 screened pregnant women had anti-SARS-CoV-2 antibodies, with seroprevalence of 7% (IC95%: 6.1% to 8.1%). Among the cases of positive seroprevalence, IgM was observed in 10% (IC95%: 6.1% to 15.8%), IgM / IgG in 78.8% (IC95%: 71.8% to 84.6%) and IgG in 11.2% cases (IC95%: 7.0% to 17.1%) (Table 1). The daily number of new cases with anti-SARS-CoV-2 antibodies remained erratic, not showing a definite trend. But, the number of cumulative cases per day was on the rise during the study period (Figure 1).
Table 1 Results of The sars-cov-2 virus serological test in pregnant women screened at hospital admission.
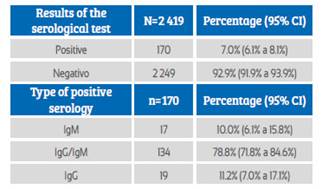
Source: own elaboration
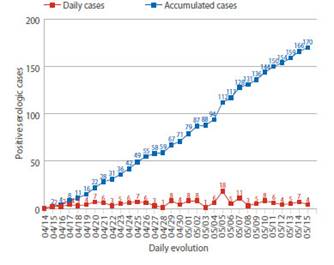
Figure 1 Daily number of new cases and accumulated cases of pregnant women with anti-sars-cov-2 antibodies at hospital admission.
Among pregnant women who tested positive to the serological test, the IgM / IgG type was most frequently observed, mainly in adulthood or at an advanced maternal age. It occurred more frequently in pregnant women during the third trimester of pregnancy with inadequate prenatal control, in primiparous and multiparous women, cohabitants, with secondary education and housewives. No association was found between these characteristics and the type of anti-SARSCoV-2 antibody response (Table 2).
Table 2 Association of epidemiological and obstetric characteristics according To The anti-sars-cov-2 antibodies Response.
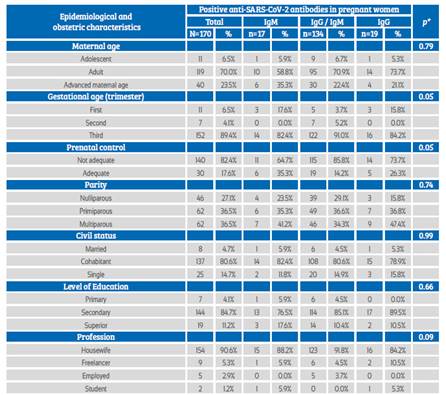
*Fisher’s exact test
Source: own elaboration
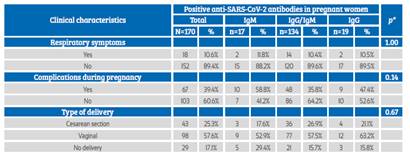
Obstetric complications occurred in 35.9% of the patients, with premature rupture of membranes, preeclampsia and spontaneous abortion being more frequent (Table 3). 89.4% of pregnant women were asymptomatic, 39.4% presented some type of clinical complications, and the most frequent type of delivery was vaginal (57.6%); no association was observed between these clinical characteristics and type of anti-SARS-CoV-2 antibody response (p>0.05) (Table 4).
Table 3 Clinical characteristics in pregnant women admitted To The hospital.
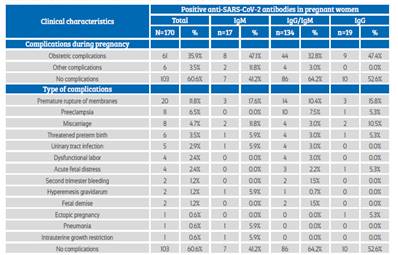
Source: own elaboration
Discussion
In a short time, diagnostic test manufacturers around the world developed molecular and immunological tests for the diagnosis of SARSCoV-2. The key criterion for the choice of these tests for procurement and use was validation by one of the world's leading bodies, such as the European Community (EC) and the US Food and Drug Administration (FDA). An EC-validated test was used in this study, ensuring that the parameters were within internationally acceptable standards.
Although serological tests are useful for studies to determine epidemiological variables of public health interest13, there are still limited reports of their application in the pregnant population.
In the present study, a 7% seroprevalence of anti-SARS-CoV-2 antibodies was observed in pregnant women admitted to the hospital. The rapid test applied met the recommendation of simultaneous detection of IgM and IgG immunoglobulins10,12. Among all pregnant women with positive result, positive IgM / IgG was most frequently observed. Studies suggest that most patients seroconvert between 7 and 11 days after exposure to the virus10,11. Our findings in the studied population would represent a possible infection of pregnant women in the community who attend a specialized center for delivery or for care of morbidities. The health professional should consider these findings when providing the corresponding comprehensive care and management.
In the group of pregnant women who tested positive for anti-SARS-CoV-2 serology, a higher frequency was observed in adulthood and older women than in adolescence, similar to the national trend14. In previous studies, it has been identified that the current pandemic is mainly affecting older patients15, which may also be one of the factors involved in the differences found between older pregnant women and pregnant adolescents. The most frequent positive pregnancies were cohabiting women with low level of education and mainly housewives. This also reflects the trend in sociocultural characteristics of the pregnant population in Peru14. The current pandemic is spreading rapidly in the Peruvian population16, so its distribution would be similar among pregnant women.
Pregnant women presented a higher frequency of positive anti-SARS-CoV-2 serological results during the third trimester, similar to recent studies3,6. One factor involved may be the current system of prenatal controls, which are more continuous only in the third trimester17, as well as to morbidities at the end of pregnancy for which pregnant women seek care17,18. COVID-19 impact on the evolution of pregnancy itself is not clear yet7.
In the study, 89.6% of the pregnant women with positive serological test were asymptomatic, and the frequency of respiratory symptoms was similar among the types of anti-SARS-CoV-2 antibody response. These findings were similar to those found by Sutton et al19 in New York, but using molecular PCR testing, in which 88% of pregnant women with COVID-19 infection admitted for labor were asymptomatic. Vintzileos et al20 found a lower proportion (66%) of asymptomatic women. On the other hand, we should consider that the performance of the test depends on a factor that is difficult to measure and predict, which is the time of sampling with respect to the time of illness, which can be calculated only in frankly symptomatic cases, but much difficult in asymptomatic or oligoasymptomatic cases, that according to research reaches 80-85% of those infected21. These results support the screening of all pregnant women admitted to hospitals, regardless of the presence or absence of respiratory symptoms, since most pregnant women do not present characteristic symptoms; this allows facilities to differentiate the respective care and isolation of positive cases.
35.9% of the pregnant women with positive SARSCoV-2 antibodies had obstetric complications, most often premature rupture of membranes, preeclampsia and miscarriage. These findings were similar to those of studies where molecular PCR tests were used to identify disease cases22. Other studies have found increased frequency of preterm delivery and intrauterine growth restriction3. Premature termination of pregnancy has also been identified, although these women also manifested concurrent complications such as preeclampsia, premature rupture of membrane, irregular contractions and history of fetal death, without being able to prove if these complications were associated with SARS-CoV-2 infection6.
In the present study, the predominant route of delivery among serologically positive pregnant women was vaginal (57.6%). We observed that the indication for cesarean section was obstetrical. In the period studied, the rate of cesarean section decreased to 25.3%, which is less than our hospital historical rate (43%) 23. This is in accordance with the review by Ashokka et al24 and with the expert consensus25, that recommend the mode of delivery be based on obstetrical indications, allowing vaginal delivery whenever possible and reserving cesarean section only for obstetrical reasons, not only because of the mother's SARS-CoV-2 infection. However, there are studies in pregnant women with SARSCoV-2 infection in which cesarean section has increased, not justified by obstetrical causes or illness severity26,27, and vaginal delivery decreased between 6.8% 26 and 9.4% 27.
Although data were collected from a large population of pregnant women, with serological testing used to screen all patients admitted to hospital, there are limitations to the study, such as the retrospective data collection and the use of the test itself. Although serological tests are not the first choice for timely diagnosis, they are used when molecular tests are not available or not easily accessible, as is the case in our population, in face of growing increase of cases that saturate health services. On the other hand, sensitivity and specificity data are variable in the literature, and this will probably depend on the kinetics of antibody production in each patient, and there is still limited literature on their evaluation in the pregnant population.
Based on these results, we conclude that in our study pregnant women with hospitalization for delivery or pregnancy morbidity care presented a seroprevalence of 7%. Most of them did not show clinical evidence of infection and were in a more evolved acute phase, presenting IgM / IgG anti-SARS-CoV-2 antibodies type. The most frequent complications were premature rupture of membranes and preeclampsia. No association was evident between the clinical-epidemiological characteristics analyzed and the type of antiSARS-CoV-2 antibody response.
REFERENCES
1. World Health Organization (WHO). Coronavirus disease (COVID-19) pandemic 2020. (acceso 10 de mayo 2020). Disponible en: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [ Links ]
2. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infectious Dis. 2020;20(5):533-4. Doi: 10.1016/S1473-3099(20)30120-1 [ Links ]
3. Dashraath P, Wong JLJ, Lim MXK, Lim LM, Li S, Biswas A, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy [published online ahead of print, 2020 Mar 23]. Am J Obstet Gynecol. 2020;S0002-9378(20)30343-4. doi:10.1016/j.ajog.2020.03.021 [ Links ]
4. Soma-Pillay P, Catherine N-P, Tolppanen H, Mebazaa A, Tolppanen H, Mebazaa A. Physiological changes in pregnancy. Cardiovasc J Afr. 2016; 27(2):89-94. doi: 10.5830/CVJA-2016-021 [ Links ]
5. Buekens P, Alger J, Bréart G, Cafferata ML, Harville E, Tomasso G. A call for action for COVID-19 surveillance and research during pregnancy. Lancet Glob Health. Published online 22 April 2020. doi:10.1038/s41586-020-2012-7 [ Links ]
6. Liu H, Wang LL, Zhao SJ, Kwak-Kim J, Mor G, Liao AH. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Reprod Immunol. 2020;139:103122. doi: 10.1016/j.jri.2020.103122 [ Links ]
7. Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet. 2020;395(10226):760-2. doi: 10.1016/s0140-6736(20)30365-2 [ Links ]
8. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727-32. doi: 10.3201/eid2203.151049 [ Links ]
9. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045 [ Links ]
10. Zhang W, Du R-H, Li B, Zheng X-S, Yang X-L, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerging microbes infections. 2020;9(1):386-9. doi: 10.1080/22221751.2020.1729071 [ Links ]
11. Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020;ciaa344. doi: 10.1093/cid/ciaa344 [ Links ]
12. Osterdahl M, Lee K, Ni Lochlainn M, Wilson S, Douthwaite S, Horsfall R, et al. Detecting SARS-CoV-2 at point of care: Preliminary data comparing loop-mediated isothermal amplification (LAMP) to PCR. MedRxiv. 2020; doi: https://doi.org/10.1101/2020.04.01.20047357 [ Links ]
13. Gómez Marin JE, Castellanos J, Rodriguez-Morales AJ, Cardona-Ospina JA, Forero Duarte JE, Mattar S, Esparza G. Consenso de grupo ad-hoc sobre recomendaciones para la evaluación y controles de calidad para el diagnóstico molecular y serológico de la infección humana por SARS CoV-2. Infectio. 2020;24(3 suppl 2). doi: http://dx.doi.org/10.22354/in.v24i3.868 [ Links ]
14. Espinola-Sanchez M, Racchumi-Vela A, Arango-Ochante P, Minaya-León P. Perfil sociodemográfico de gestantes en el Perú según regiones naturales. Rev Peru Investig Matern Perinat. 2019;8(2):14-20. doi: https://doi.org/10.33421/inmp.2019149 [ Links ]
15. Wang D, Yin Y, Hu C, Liu X, Zhang X, Zhou S, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two Hospitals in Wuhan, China. Crit Care. 2020;24(1):188. doi: 10.1186/s13054-020-02895-6 [ Links ]
16. Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:411-5. doi:http://dx.doi.org/10.15585/mmwr.mm6914e1 [ Links ]
17. Tipiani O, Tomatis C. El control prenatal y el desenlace maternoperinatal. Rev Per Ginecol Obstet. 2006;52(4):46-8. doi: 10.31403/rpgo.v52i319 [ Links ]
18. Gonzales-Carrillo O, Llanos-Torres C, Espinola-Sánchez M, Vallenas-Campos R, Guevara-Ríos E. Morbilidad materna extrema en mujeres peruanas atendidas en una institución especializada. 2012-2016. Rev Cuerpo Med HNAAA. 2020;13(1):8-13. doi: 10.35434/rcmhnaaa.2020.131.594 [ Links ]
19. Sutton D, Fuchs K, D'Alton M, Goffman D. Universal sscreening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;NEJMc2009316. doi: 10.1056/NEJMc2009316 [ Links ]
20. Vintzileos WS, Muscat J, Hoffmann E, Vo D, John NS, Vertichio R, et al. Screening all pregnant women admitted to labor and delivery for the virus responsible for COVID-19. Am J Obstet Gynecol. Published online: April 26, 2020. doi: 10.1016/j.ajog.2020.04.024 [ Links ]
21. Luque N, Salcedo C. COVID-19 y las unidades de cuidados intensivos en el Perú. Rev med intens cuidados críticos. 2020;13(1):40-4. [ Links ]
22. Guan W, Ni Z, Hu Y, Liang W, Ou Ch, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;80(6):656-65 doi: https://doi.org/10.1016/j.jinf.2020.03.041 [ Links ]
23. Instituto Nacional Materno Perinatal. Oficina de Estadística e Informática. Boletín Estadístico 2018 [Internet]. Perú. 2018 [citado 12 de mayo de 2020]. Disponible en: https://www.inmp.gob.pe/institucional/boletines-estadisticos/1422371837 [ Links ]
24. Ashokka B, Loh M-H, Tan CH, Su LL, Young BE, Lye DC, et al. Care of the pregnant woman with COVID-19 in labor and delivery: anesthesia, emergency cesarean delivery, differential diagnosis in the acutely ill parturient, care of the newborn, and protection of the healthcare personnel. Am J Obstet Gynecol. Published online: April 10, 2020. Doi: https://doi.org/10.1016/j.ajog.2020.04.005 [ Links ]
25. Chen D, Yang H, Cao Y, Cheng W, Duan T, Fan C, et al. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID-19) infection. Int J Gynaecol Obstet. 2020;149(2):130-6. doi: 10.1002/ijgo.13146 [ Links ]
26. Mullins E, Evans D, Viner RM, O'Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586-92. doi: 10.1002/uog.22014 [ Links ]
27. Parazzini F, Bortolus R, Mauri PA, Favilli A, Gerli S, Ferrazzi E. Delivery in pregnant women infected with SARS-CoV-2: A fast review. Int J Gynaecol Obstet. Published online: 9 April 2020. doi: 10.1002/ijgo.13166 [ Links ]
6This publication is original and has not been previously published by another institution and/or scientific journal. Study approved by the Institutional Ethics Committee and has the institution permission
Funding: the authors certify that they have not received specific financial support, equipment or materials from private persons, public and/or private institutions to perform this study.
10Cite as: Guevara-Ríos E, Espinola-Sánchez M, Carranza-Asmat C, Ayala-Peralta F, Álvarez-Carrasco R, Luna-Figueroa A, Meza-Santibáñez L, Pérez-Aliaga C, Zevallos-Espinoza K, Racchumi-Vela A, Segundo-Paredes J, Arango-Ochante P. Anti-SARS-CoV-2 antibodies in pregnant women at a level III hospital in Peru. Rev Peru Ginecol Obstet. 2020;66(3). DOI: https://doi.org/10.31403/rpgo.v66i2259
Received: May 26, 2020; Accepted: June 24, 2020











 text in
text in