Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.3 Lima Jul-Sep 2020
http://dx.doi.org/10.31403/rpgo.v66i2276
Short Communication
Clinical characteristics of pregnant women in labor with SARS-CoV-2 infection at high altitude: A case series
1 Department of Obstetrics and Gynecology, Hospital Regional Docente de Cajamarca, Peru
2 Department of Obstetrics and Gynecology, Simón Bolívar COVID-19 Hospital, Cajamarca, Peru
3 Department of Obstetrics and Gynecology, Medimagen, Cajamarca, Peru
4 Department of Obstetrics and Gynecology, Complejo Hospitalario Dr. Arnulfo Arias Madrid Hospital, Caja del Seguro Social, Panama
5 National Institute of Allergy and Infectious Diseases, Maryland, EE UU
Introduction:
Fewer COVID-19 cases and less lethality have been observed at high altitude compared to cases reported at sea level. There are currently no publications reporting clinical behavior of pregnant women with COVID-19 at high altitude.
Methods:
This is a retrospective study with review of medical records between March 6, 2020 and June 15, 2020. The first thirteen cases of pregnant women with COVID-19 who were attended at Simón Bolívar COVID-19 Hospital, located at 2 750 meters above sea level, are described. The cases came from altitudes between 2 035 and 3 502 meters above sea level (masl). Statistical analysis used SPSS, version 19.0.
Results:
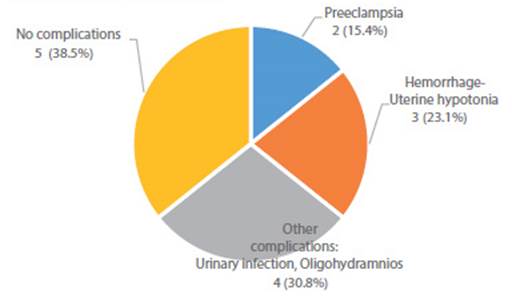
Thirteen cases of pregnant women with COVID-19 confirmed by IgM for SARS-CoV-2 were attended at 2 750 masl (9 022.31 feet) in the Peruvian Andes. Delivery by cesarean section occurred in eight cases (61.5%) and five (38.5%) delivered vaginally. There were two cases (15.4%) of preeclampsia, one with diagnosis of HELLP syndrome, prematurity and fetal death. Three cases (23.1%) developed uterine hypotonia that required Hayman or B-Lynch suture. Two cases (15.38%) were complicated with oligohydramnios and two with urinary infection. Hemoglobin levels were between 11.1 and 16 g/dL. Only one case (7.7%) was symptomatic, with mild pharyngeal pain. No vertical transmission was detected by IgM/IgG for SARS-CoV-2. Clinical evolution was favorable in the thirteen cases and they were discharged after 2 to 4 days hospitalization to continue home quarantine.
Conclusions:
Results in this short study show pregnant women in labor with COVID-19 by rapid IgM test for SARS-CoV-2 at high altitude were mostly asymptomatic; there was no vertical transmission, but high presence of other obstetrical complications.
Key words: Pregnancy; Coronavirus infections; COVID-19; SARS CoV-2; High Altitude; Preeclampsia; Cajamarca; Peru.
Introduction
Fewer cases and less lethality from COVID-19 have been seen at high altitudes compared with the numbers reported at sea levels1. Climatological and demographic factors could explain this observation2,3. At high altitude, chronic hypoxia has resulted in the genetic adaptation of man to a reduced amount of oxygen4-8. The association between hypoxia and a decreased expression of angiotensin-converting enzyme 2 (ACE2) is known1. ACE2 is the main receptor of the SARSCoV-2 virus9. In addition, an increase in the activity and expression of ACE2 during pregnancy has been reported in experimental studies10. In Spain, the seroprevalence for SARS-CoV-2 during pregnancy has been published in 14%11.
For diagnosis, viral RNA detection tests and serology tests that detect IgG/IgM antibodies are used12.
Clinically, the majority of pregnant women are asymptomatic (87.9%)13, and as for pregnant women with symptoms, they have mild (86%), severe (9.3%) and critical (4.7%) illness14. The most frequent symptoms in pregnant women with SARS-CoV-2 are cough (76%) and fever (38%)15.
Complications from COVID-19 mostly occur in the third trimester, including 4.2% of patients who suffer from pneumonia11. Obstetric complications are observed in 45% of cases15. Clinical findings resembling preeclampsia have been reported16-18. Regarding the mode of delivery, 88% is via cesarean section15.
Vertical transmission during pregnancy, childbirth and the puerperium is very low, despite being SARS-CoV-2 found in the placenta, not so in cord blood or breast milk19,20.
At present, no publications have been found that indicate the clinical behavior of COVID-19 in pregnant women adapted to altitude and chronic hypoxia.
Our objective is to describe the maternal and neonatal clinical characteristics of the first cases of women in labor seen in the highlands of Peru infected with COVID-19 and diagnosed by rapid IgM or IgM/IgG antibodies tests.
Methods
The present is a retrospective study of a series of cases of pregnant women treated in a single center located in the altitude between March 6, 2020 and June 15, 2020. It was approved by the institutional board of directors and informed consent was obtained from the pregnant women. We analyzed the clinical charts of 13 pregnant women admitted for labor in the city of Cajamarca, Peru, at 2 750 masl.
The tests used for the detection of antibodies against SARS-CoV-2 were subjected to immunochromatographic assay for the rapid, qualitative, and differential detection of IgG and IgM antibodies, with a sensitivity of 91.8% and specificity of 96.4%.
For the study, we used the CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development checklist. Statistical analysis was performed with SPSS software, version 19.0. Continuous variables were expressed directly as a range. Categorical variables were expressed as numbers (%).
Results
The 13 pregnant women in labor were attended at Simón Bolívar COVID-19 Hospital in Cajamarca, Perú, located at 2 750 masl. They came from altitudes between 2 035 and 3 502 masl. All cases were positive for SARS-CoV-2 IgM or IgM/IgG. Age ranged from 19 to 41 years, gestational age from 33 to 41 weeks. One patient (7.7%) presented mild symptoms (pharyngeal pain) and twelve (92.3%) were asymptomatic. Type of delivery was cesarean section in 8 (61.5%) cases and vaginal in 5 (38.5%). Eight (61.5%) had other pregnancy complications and five (38.5%) did not; two (15.4%) had preeclampsia, one (7.7%) of them HELLP syndrome that resulted in prematurity and fetal death. Three (23.1%) were complicated by postpartum hemorrhage due to uterine hypotonia during cesarean section, which required Hayman or B-Lynch suture. Two (15.4%) had oligohydramnios and other two (15.4%) urinary tract infection (Table 1, Figure 1). No patient required admission to intensive care unit or received specific treatment for COVID-19.
Table 1 Cities of origin, altitude and clinical characteristics
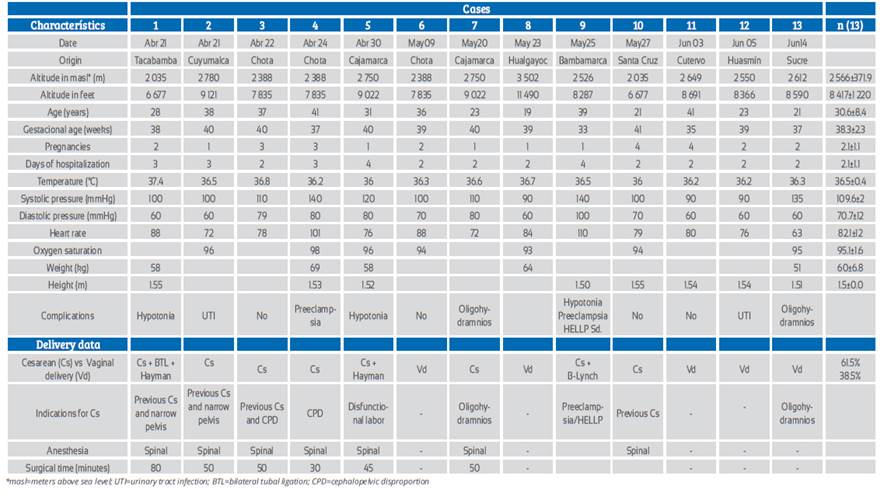
*masl=meters above sea level; UTI=urinary tract infection; BTL=bilateral tubal ligation; CPD=cephalopelvic disproportion
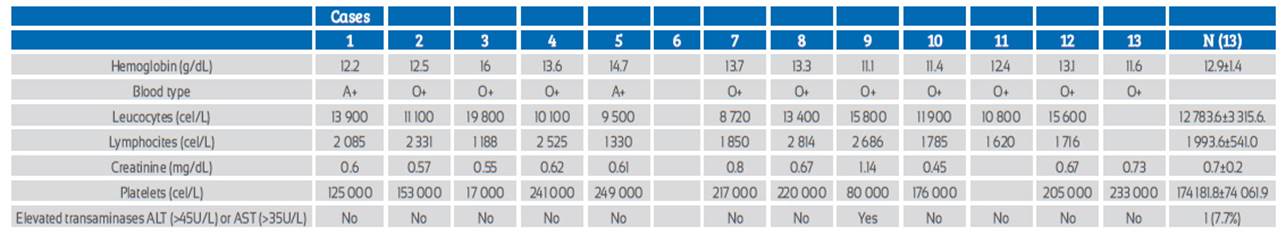
Regarding the laboratory characteristics (Table 2), hemoglobin was reported between 11.1 g/dL and 16 g/dL, leucocytes between 8 720 /mL and 19 800 /mL, lymphocytes 1 188/mL to 2 814/mL, and platelets between 80 000/mL and 249 000/mL.
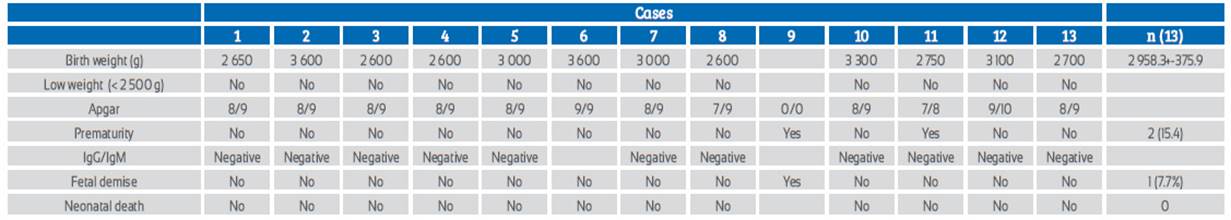
All newborns had a negative rapid test for SARSCoV-2 IgM/IgG. The stillborn was not tested. All newborns were breastfed. The weights of the newborns ranged between 2 600 and 3 600 g. There were two cases of prematurity (15.4%) and one case of fetal death (7.7%) (Table 3).
Discussion
92.3% of pregnant women evaluated in labor coming from altitudes between 2 035 and 3 502 masl, with positive IgM antibodies by rapid test for SARS-Cov-2 had asymptomatic or mild disease, and 61.5% suffered obstetric complications. There were 15.4% prematurity and 7.7% fetal deaths. No vertical transmission was found.
In altitude, most of the pregnant women studied had asymptomatic or mild disease, similar to studies from the USA, i.e., approximately 87%13,14. The cesarean delivery rate was lower than worldwide, i.e. 61.5% instead of 88%15. However, the high percentage of other obstetrical complications associated to the COVID-19 virus stands out, as it surmounts to 61,5% instead of the 45% published in a systematic review15. Preeclampsia (15.4%) and uterine atony (23.0%) were the complications found. There was no vertical transmission detected by IgG/IgM in neonates, data that coincides with the report by Chen, who found no vertical transmission19.
Limitations of this study are having few cases, being retrospective, not having carried out molecular tests and having only evaluated patients during the third trimester; reasons for which the conclusions should be considered in context.
These results show that pregnant women in labor with a rapid test for SARS-CoV-2 in a highland region of Peru were generally asymptomatic or had mild disease. No vertical intrauterine transmission was found and there was a high percentage of other obstetric complications.
REFERENCES
1. Arias-Reyes C, Zubieta-DeUrioste N, Poma-Machicao L, Aliaga-Raudan F, Carvajal-Rodríguez F, Durschmann M, et al. Does the pathogenesis of SARS-CoV-2 virus decrease at high-altitude? Respir Physiol Neurobiol. 2020 Apr;277:103443. doi:10.1016/j.resp.2020.103443 [ Links ]
2. Ahmadi MF, Sharifi A, Dorosti S, Jafarzadeh Ghoushchi S, Ghanbari N. Investigation of effective climatology parameters on COVID-19 outbreak in Iran [published online ahead of print 2020]. Sci Total Environ. 2020;729:138705. doi:10.1016/j.scitotenv.2020.138705 [ Links ]
3. Bashir MF, Ma B, Bilal, Komal B, Mashir MA, Tan D, Bashir M. Correlation between climate indicators and COVID-19 pandemic in New York, USA. Sci Total Environ. 2020;728:138835. doi:10.1016/j.scitotenv.2020.138835 [ Links ]
4. Julian CG, Moore LG. Human genetic adaptation to high altitude: Evidence from the Andes. Genes. 2019;10(2):1-20. doi: 10.3390/genes10020150 [ Links ]
5. Moore LG. Measuring high-altitude adaptation. J Appl Physiol 2017 Nov 1;123(5):1371-85. doi: 10.1152/japplphysiol.00321.2017 [ Links ]
6. Hamming I, Cooper ME, Haagmans BL, Hooper NM, Korstanje R, Osterhaus ASME, et al. The emerging role of ACE2 in physiology and disease. J Pathol. 2007 May;212(1):1-11. doi: 10.1002/path.2162 [ Links ]
7. Bigham AW, Kiyamu M, León-Velarde F, Parra E, Rivera-Ch M, Shriver M, Brutsaert T. Angiotensin-converting enzyme genotype and arterial oxygen saturation at high altitude in Peruvian Quechua. High Alt Med Biol. 2008;9(2):167-78. [ Links ]
8. Vargas M, Vargas E, Julian CG, Armaza JF, Rodriguez A, Tellez W, mNiermeyer S, et al. Determinants of blood oxygenation during pregnancy in Andean and European residents of high altitude. Am J Physiol Regul Integr Comp Physiol. 2007 Sep;293(3):R1303-12. DOI: 10.1152/ajpregu.00805.2006 [ Links ]
9. Li Y, Zhou W, Yang L, You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res. 2020;157:104833. doi:10.1016/j.phrs.2020.104833 [ Links ]
10. Levy A, Yagil Y, Bursztyn M, Barkalifa R, Scharf S, Yagil C. ACE2 expression and activity are enhanced during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1953-R1961. [ Links ]
11. Crovetto F, Crispi F, Llurba E, Figueras F, Gómez-Roig MD, Gratacós E. Seroprevalence and presentation of SARS-CoV-2 in pregnancy [published online ahead of print, 2020 Aug 6]. Lancet. 2020;S0140-6736(20)31714-1. doi:10.1016/S0140-6736(20)31714-1 [ Links ]
12. Ravi N, Cortade DL, Ng E, Wang SX. Diagnostics for SARSCoV-2 detection: A comprehensive review of the FDA-EUA COVID-19 testing landscape [published online ahead of print, 2020 Jul 18]. Biosens Bioelectron. 2020;165:112454. doi:10.1016/j.bios.2020.112454 [ Links ]
13. Sutton D, Fuchs K, D'Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020 May 28;382(22):2163-4. DOI: 10.1056/NEJMc2009316. [ Links ]
14. Breslin N, Baptiste C, Gyamfi-Bannerman C, Miller R, Martinez R, Bernstein K, et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020 May;2(2):100118.doi: 10.1016/j.ajogmf.2020.100118 [ Links ]
15. Capobianco G, Saderi L, Aliberti S, Mondoni M, Piana A, Dessole F, et al. COVID-19 in pregnant women: A systematic review and meta-analysis [published online ahead of print, 2020 Jul 16]. Eur J Obstet Gynecol Reprod Biol. 2020 Jul 16;S0301-2115(20)30446-2. doi:10.1016/j.ejogrb.2020.07.006 [ Links ]
16. Mendoza M, Garcia-Ruiz I, Maiz N, et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study [published online ahead of print, 2020 Jun 1]. BJOG. 2020 Jun 1;10.1111/1471-0528.16339. doi:10.1111/1471-0528.16339 [ Links ]
17. Abbas AM, Ahmed OA, Shaltout AS. COVID-19 and maternal pre-eclampsia: A synopsis [published online ahead of print, 2020 Jun 15]. Scand J Immunol. 2020;e12918. doi:10.1111/sji.12918 [ Links ]
18. Rolnik DL. Can COVID-19 in pregnancy cause pre-eclampsia? [published online ahead of print, 2020 Jun 22]. BJOG. 2020;10.1111/1471-0528.16369. doi:10.1111/14710528.16369 [ Links ]
19. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809-815. DOI:https://doi.org/10.1016/S0140-6736(20)30360-3 [ Links ]
20. Lackey KA, Pace RM, Williams JE, Bode L, Donovan SM, Järvinen KM, et al. SARS-CoV-2 and human milk: What is the evidence? [published online ahead of print, 2020 May 30]. Matern Child Nutr. 2020;e13032. doi:10.1111/mcn.13032 [ Links ]
Received: August 17, 2020; Accepted: August 22, 2020











 text in
text in