Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.3 Lima Jul-Sep 2020
http://dx.doi.org/10.31403/rpgo.v66i2263
Case Report
Recurrent catamenial pneumothorax. Case report
1. Department of Obstetrics and Gynecology. Central Hospital “Dr. Urquinaona”, Maracaibo, Zulia State, Venezuela
Catamenial pneumothorax is a rare and complex clinical condition that should be considered as a cause of spontaneous and recurrent pneumothorax that is often misdiagnosed. It is a pulmonary pathology commonly associated with menstruation since it occurs within 72 hours before or after the onset of menstrual bleeding. The etiology and the exact underlying mechanism have not been identified, but it could be a rare form of extra-pelvic endometriosis characterized by the presence of functional endometrial tissue in the pleura, pulmonary parenchyma, and respiratory tract. Diagnosis is a challenge, so it can result in recurrences. It should be suspected in young women of childbearing age. The first line of treatment is medical, while surgical treatment is necessary to avoid recurrence. A case of recurrent catamenial pneumothorax is presented.
Key words: Catamenial pneumothorax; Endometriosis; Thoracic endometriosis; Pneumothorax
Introducción
Catamenial pneumothorax, although rare, is considered a common cause of recurrent spontaneous pneumothorax1. It is the most common form of the thoracic endometriosis syndrome and its main characteristic is presence of air in pulmonary areas as a result of endometriotic implants in the pleura, lung parenchyma and/or airways. This allows air and blood to cause pulmonary collapse. It usually occurs the day before or 2 or 3 days after menstruation2.
Clinical manifestations of catamenial pneumothorax include shortness of breath, dyspnea, pleuritic pain and, rarely, hemoptysis3. Notwithstanding years of description, the pathophysiology is completely unknown; considered a mysterious disease, it is difficult to diagnose and treat2. We report a case of recurrent catamenial pneumothorax.
Clinical case
A 34-year-old patient, III gestations, II paras, I abortion, presented a spontaneous episode of right-sided pleuritic chest pain, shortness of breath (which progressively worsened with physical activity) and dry cough, that appeared two days after starting menstruation. She reported a history of three episodes of right spontaneous pneumothorax that also coincided with her menstrual period and spontaneous complete remission with rest. Menarche was at age 14, 28 days menstrual cycles, 6 days of bleeding with dysmenorrhea and dyspareunia since age 20 years. The patient had undergone dilatation and curettage one year earlier for incomplete abortion. She denied history of trauma, cough, fever, smoking, or chronic lung disease.
The patient was in her third day of menstrual cycle, in regular general conditions, afebrile and stable vital signs (blood pressure 139/81 mmHg, heart rate 91 beats per minute, respiratory rate 18 beats per minute, and blood oxygen saturation 96%). The trachea did not show deviation, but there was decreased thoracic expansion, asymmetric respiratory movements, and the left thoracic breath sounds audible on auscultation, but absent in the right thorax. Blood tests, basic blood chemistry, and venous blood gases were within normal limits.
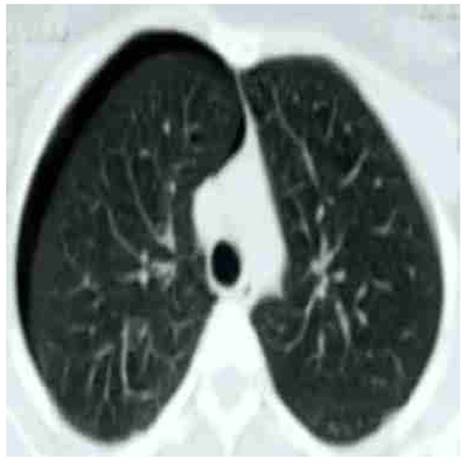
Chest X-ray showed right pneumothorax associated with nebulous areas in the subpleural anterior basal segment, with no displacement of the mediastinum (Figure 1). Chest computed to mography confirmed these findings and showed small, ground-glass opacity nodules in the diaphragm, with no evidence of bronchiectasis or emphysematous bullae (Figure 2). The patient was hospitalized and underwent thoracotomy. A 28 FG size chest tube was inserted at the midpoint of the fourth intercostal space between the anterior and mid-axillary line, to alleviate symptoms. Due to the multiple recurrences and the clinical and radiological findings, the possibility of thoracic endometriotic foci was considered. Transvaginal ultrasound showed the uterus and ovaries morphologically normal, with small nodular lesions in the rectovaginal septum, possible endometriotic. CA-125 concentrations were 75 IU / L (normal value up to 35 IU / L).
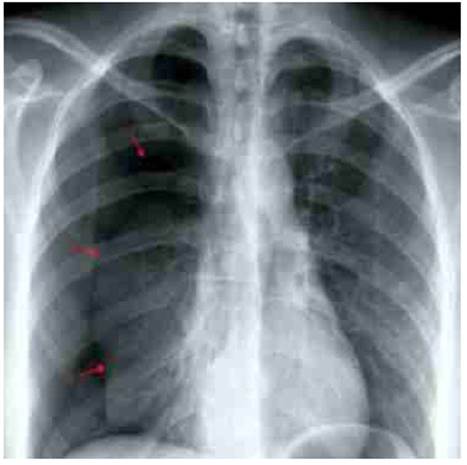
Figure 1 plain Chest x-ray showing right pneumothorax. arrows indiCate the edge oF the lung silhouette.
To avoid new recurrences, the patient was referred to the thoracic surgery service and scheduled for video-assisted thoracoscopic surgery. After entering the right pleural space, multiple small pores were observed in the diaphragm, and small macroscopic reddish lesions compatible with endometriotic implants, on the pleural and diaphragmatic surfaces (Figure 3). During the procedure, diaphragmatic spaces were closed with 2-0 vicryl, implants were resected with electrocautery, and pleurodesis with sterile talc diluted in physiological solution was done.
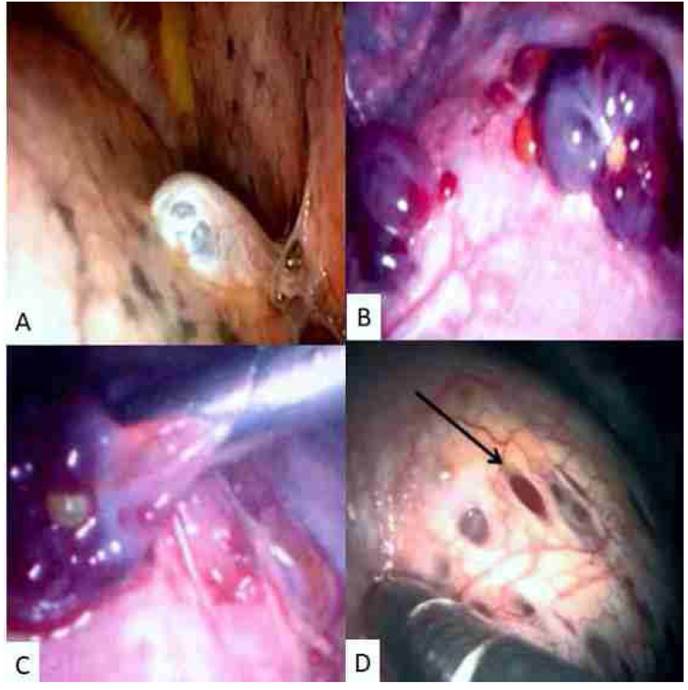
Figure 3 Intraoperative videothoracoscopy findings. A) Presence of several milimetric lesions in pleura and diaphragm. B) Endometrial implants on the diaphragmatic surface. C) Resection of endometrial implants. D) Presence of diaphragmatic pores.
Histological evaluation of the lesion showed dilated and hyperplastic endometrial glands, with abundant collapsed hemorrhagic endometrial stroma along with cystic glands aligned in a monolayer of cuboidal epithelium and perivascular infiltration of lymphocytes (Figure 4). Immunohistochemical examination was positive for CK7 staining in endometrial glands and for CD10 in the endometrial stroma, along with positivity for estrogen and progesterone receptors in the glandular and stromal cell nuclei. Ki 67 score was 5% for stroma and 5% to 30% for epithelial cells. No signs of endometrial hyperplasia, atypia or malignancy were found. These findings confirmed the diagnosis of catamenial syndrome secondary to endometriotic implants in lungs and diaphragm.
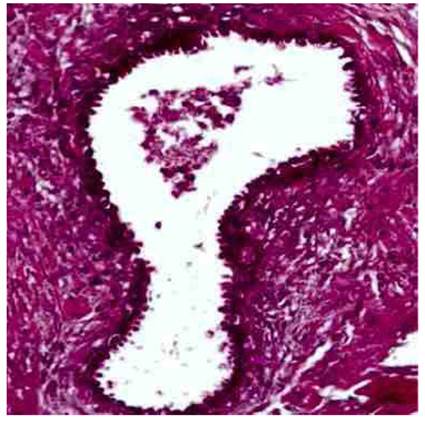
Figure 4 Microscopic image of the lesion; presence of stroma and endometrial-type glands (40X hematoxylin-eosin staining).
The patient remained hospitalized in stable conditions and without episodes of pain. She was discharged on the fifth postoperative day, starting treatment with monthly gonadotropin-releasing hormone analogs for 3 months. During follow-up, patient has not presented recurrence of symptoms, and CA-125 concentrations have remained within normal values.
Discussion
Endometriosis is the presence of endometrial tissue outside the uterine cavity. It is usually found in the pelvis, but it can occur anywhere in the body, including abdomen, chest, skin, and central nervous system4. Between 5% and 10% of women of reproductive age and 2% to 5% of menopausal women are diagnosed with this condition. Thoracic endometriosis is rare. Its prevalence has been underestimated in the literature until the last decade5.
Thoracic endometriosis syndrome is a pathology where ectopic endometrial tissue is deposited in the thoracic structures and is characterized by catamenial pneumothorax (73%), catamenial hemothorax (14%), catamenial hemoptysis (7%) and pulmonary nodules in relation to menstrual cycle, but without clear histological evidence6. Pelvic endometriosis is present in 30% to 50% of cases. Pneumothorax can be catamenial and non-catamenial. Those of the catamenial type are the most common, accounting for 31% to 35% of cases; those of non-catamenial pneumothorax are difficult to diagnose and can generally only be identified by surgery7.
Despite association between pneumothorax and menstruation, researchers have not been able to find explanation for the pathophysiology. There are several theories to explain the cause of this rare clinical condition1. The first theory is trans-diaphragmatic passage of air from the vagina, via the fallopian tubes to the peritoneum, reaching the thoracic cavity through diaphragmatic defects or porosities. Absence of a cervical mucous plug allows communication between the peritoneal cavity and the exterior through the uterine corpus and fallo-pian tubes. The second theory is active air leakage from endometrial implants located in the pleura. Another theory is derived from high concentrations of prostaglandins during the menstrual period. This causes both vascular and bronchial vasoconstriction, leading to traumatic injury and alveolar rupture, that would eventually result in air leakage8-10.
Catamenial pneumothorax may go unnoticed. For this reason, it is important to obtain a complete medical history and physical examination in all patients with recurrent spontaneous pneumothorax. The most common clinical symptoms include chest pain (occurs more frequently, in 90% of cases), followed by dyspnea (31%), hemoptysis (7%), and cough (rare), that generally occur within 72 hours from the beginning of menstruation2,6. Symptoms of associated pelvic endometriosis can be mild and go unnoticed, so diagnosis may be difficult. Physical examination may show decreased or absent breath sounds on the affected side.
Catamenial pneumothorax can be differentiated from spontaneous pneumothorax by four clinical elements: right-sided pneumothorax, history of pelvic endometriosis, age 31 years or older, and absence of history of smoking. History of hemoptysis during the menstrual period can be an indicator of pulmonary endometriosis. These characteristics have a high predictive value for differentiating pneumothorax related to thoracic endometriosis syndrome from a spontaneous pneumothorax11.
Possible explanation for more affection of the right hemithorax may be the clockwise movement of endometrial tissue within the peritoneal fluid, from the pelvis along the right paracolic canal to the subphrenic space. Two forces would contribute to this movement: the falciform ligament would prevent movement to the left, and changes in intraperitoneal pressure during breathing cause the right hemidiaphragm to contract on the liver. This generates a piston-like effect, enhancing movement of the endometrial tissue below the right hemidiaphragm12. However, there are reports of pneumothorax in the left hemidiaphragm, and bilaterally13.
Initial study for catamenial pneumothorax includes chest X-ray that will show the pneumothorax, pleural effusions, pulmonary nodules and, in some cases, pneumoperitoneum. High-resolution computed tomography of the chest helps to provide information on lesions such as ground-glass opacities or nodules. Magnetic resonance is more sensitive than computed tomography; it detects blood products within endometrial deposits and blood during menstruation (hyperintensity in T2 gradient echo), but it has lower spatial resolution. Bronchoscopy denotes lesions located in the airways, but cannot detect lesions located in the lung parenchyma, pleura or diaphragm6.
Typical histological findings consist of both proliferative endometrial-like glands and endometrial stroma, the presence of which is indication of endometriosis11. Findings include inflammation and macrophages loaded with hemosiderin along with lymphocytes, plasma cells, and macrophages. Mesothelial cells are modified by inflammation, and fibrosis may be observed.
Initial therapeutic procedures include oxygen support, observation, and rest if lung collapse is small. In cases of medium or large collapse, manual or chest tube aspiration may be necessary(6). Despite multiple theories on the origin of catamenial pneumothorax, it is difficult to decide a long-term treatment. The primary goal is to eliminate acute symptoms by aspiration of air from the pleural space and placement of a thoracotomy tube. The secondary objective is prevention of recurrences by surgery and/or ovarian hormonal suppression. Recurrences can be intensively treated with sclerosing agents. Several surgical procedures include pleural ablation, resection of apical bullae and parenchymal implants, resection of diaphragmatic implants, and closure of diaphragmatic pores14
Quality of diagnosis has improved with video-assisted thoracoscopic surgery. Both diagnosis and surgical treatment related to catamenial pneumothorax may be easily accomplished with this technique7. However, surgery or medical treatment only leads to suboptimal response rates(15).
Different studies show these cases may be treated with gonadotropin-releasing hormone (GnRH) agonists, danazol, and dienogest15. GnRH agonists act by modifying the hypothalamus-pituitary-ovary axis feedback, reducing secretion of luteinizing and follicle-stimulating hormone, and producing effects of pseudomenopause or medical oophorectomy by decreasing estrogen production, that lead to inactivation and degeneration of endometriotic implants. Danazol, a synthetic androgen, causes alteration of estro-gen metabolism. Dienogest is an oral progestin that decreases estrogen production. GnRH agonists are as effective as danazol in treating pelvic endometriosis with fewer side effects14.
In conclusion, catamenial pneumothorax is a rare but important cause of recurrent pneumothorax in women of reproductive age. Diagnosis is difficult due to inconsistent clinical and radiological findings. The condition is usually secondary to the thoracic endometriosis syndrome consisting in endometriotic tissue present in the chest, usually in the diaphragm. The only temporary association that leads to diagnosis is chest pain and dyspnea during menstruation. Recurrence can be controlled with early diagnosis and treatment of pelvic endometriosis.
REFERENCES
1. Junejo SZ, Singh Lubana S, Shina SS, Tuli SS. A case of thoracic endometriosis syndrome presenting with recurrent catamenial pneumothorax. Am J Case Rep. 2018;19:573-6. doi: 10.12659/AJCR.907964 [ Links ]
2. Aissa S, Benzarti W, Alimi F, Gargouri I, Salem HB, Aissa A, et al. Catamenial pneumothorax revealing diaphragmatic endometriosis: a case report and revue of literature. Pan Afr Med J. 2017;27:112. doi: 10.11604/pamj.2017.27.112.8007 [ Links ]
3. Saito T, Saito Y, Fukumoto KJ, Matsui H, Nakano T, Taniguchi Y, et al. Clinical and pathological characteristics of spontaneous pneumothorax in women: a 25-year single-institutional experience. Gen Thorac Cardiovasc Surg. 2018;66(9):51622. doi: 10.1007/s11748-018-0952-8 [ Links ]
4. Visouli AN, Darwiche K, Mpakas A, Zarogoulidis P, Papagiannis A, Tsakiridis K, et al. Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature. J Thorac Dis. 2012;4 Suppl 1:17-31. doi: 10.3978/j.issn.2072-1439.2012.s006 [ Links ]
5. Chamié LP, Blasbalg R, Pereira RM, Warmbrand G, Serafini PC. Findings of pelvic endometriosis at transvaginal US, MR imaging, and laparoscopy. Radiographics. 2011;31(4):E77-100. doi: 10.1148/rg.314105193 [ Links ]
6. Voláková E, Kolek V, Kufa J, Zatloukal J, Chudacek J, Szkorupa M, et al. Catamenial pneumothorax - case reports and literature review. Ceska Gynekol. 2017;82(4):308-12. [ Links ]
7. Alifano M. Catamenial pneumothorax. Curr Opin Pulm Med. 2010;16(4):381-6. doi: 10.1097/MCP.0b013e32833a9fc2 [ Links ]
8. Nezhat C, Lindheim SR, Backhus L, Vu M, Vang N, Nezhat A, et al. Thoracic endometriosis syndrome: A review of diagnosis and management. JSLS. 2019;23(3). pii: e2019.00029. doi: 10.4293/JSLS.2019.00029 [ Links ]
9. Inam H, Inam S, Tahir M. Catamenial pneumothorax: A case report. J Pak Med Assoc. 2016;66(10):1327-9. [ Links ]
10. Makhija Z, Marrinan M. A case of catamenial pneumothorax with diaphragmatic fenestrations. J Emerg Med. 2012;43(1):e1-3. doi: 10.1016/j.jemermed.2009.05.023 [ Links ]
11. Haga T, Kataoka H, Ebana H, Otsuji M, Seyama K, Tatsumi K, et al. Thoracic endometriosis-related pneumothorax distinguished from primary spontaneous pneumothorax in females. Lung. 2014;192(4):583-7. doi: 10.1007/s00408-014-9598-1 [ Links ]
12. Meyers MA. Distribution of intra-abdominal malignant seeding: dependency on dynamics of flow of ascitic fluid. Am J Roentgenol Radium Ther Nucl Med. 1973;119(1):198-206. [ Links ]
13. Takahashi R, Kurihara M, Mizobuchi T, Ebana H, Yamanaka S. Left-sided catamenial pneumothorax with thoracic endometriosis and bullae in the alveolar wall. Ann Thorac Cardiovasc Surg. 2017;23(2):108-12. doi: 10.5761/atcs.cr.16-00112 [ Links ]
14. Sugimura K, Sasaki O, Shinoda M, Kawasaki S, Shinkai M. Catamenial pneumothorax: a cause of monthly breathlessness. Lancet. 2019;394(10202):952. doi: 10.1016/S0140-6736(19)32094-X [ Links ]
15. Ichiki Y, Nagashima A, Yasuda M, Takenoyama M, Toyoshima S. Surgical treatment of catamenial pneumothorax: Report of three cases. Asian J Surg. 2015;38(3):180-5. doi: 10.1016/j.asjsur.2013.09.014 [ Links ]
6Acknowledgment of authorship: All authors declare that they have contributed to the idea, study design, data collection, data analysis and interpretation, critical review of the intellectual content, and final approval of the submitted manuscript.
7Ethical responsibilities: Protection of human subjects: The authors declare that the procedures performed were in accordance with the ethical provisions of the corresponding human research ethics committee, as well as the World Medical Association and the Declaration of Helsinki.
8Data confidentiality: Authors declare that they have followed the protocols issued by the Hospital Central "Dr. Urquinaona" regarding publication of patient’ data.
9Right to privacy and informed consent: The authors obtained informed consent from all patients and/or subjects present in the paper. This corresponding author is in possession of these documents.
Received: February 07, 2020; Accepted: February 28, 2020











 text in
text in