Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.3 Lima jul-sep 2020
http://dx.doi.org/10.31403/rpgo.v66i2266
Case Report
Isolated aberrant right subclavian artery. A case report
1. Gynecology and Obstetrics Service, Hospital de Hellín, Albacete, España
An aberrant right subclavian artery (ARSA) is the most common branch abnormality of the aortic arch. It can be identified by ultrasound scan in 1% of cases. The probability of association with cardiac and/or extracardiac anomalies, as well as chromosomal abnormality, is high. Prevalence of ARSA with Down syndrome is approximately 20%, and this marker may contribute to counseling on Down syndrome during the second trimester and maybe in the first trimester. When ARSA is found, recommendations include a detailed study of the fetal anatomy for other markers of aneuploidy and to obtain a fetal echocardiogram. Invasive studies will be limited to those situations where, in addition to ARSA, other markers or other conditions that increase the risk of Down syndrome are found. However, the finding of an isolated ARSA increases the risk to zero and is considered a normal variant.
Key words: Aberrant right subclavian artery (ARSA); Down syndrome
Introduction
The aberrant right subclavian artery (ARSA) is the most frequent abnormality of the aortic arch, present in approximately 1.2% of postnatal studies and in up to 2% in prenatal series. This anatomic variant has gained special importance because it is associated with congenital heart disease and as a probable marker for either Down syndrome1, 22q11.2 deletion syndrome (Di George syndrome) and other genetic syndromes associated with abnormalities of the aortic arch2,3.
In normal conditions, the aortic arch undergoes a complex development during early pregnancy that usually results in the formation of the left aortic arch, where three main branches originate: the first branch is the brachiocephalic trunk (BT) or innominate artery, which branch off into the right subclavian artery (RSA) and the right common carotid artery (RCCA); the second branch is the left common carotid artery; and the third is the left subclavian artery4.
The development of the aortic arch is abnormal in approximately 1% to 2% of fetuses2-4. Sometimes the disruption of the double aortic arch occurs between the RCCA and the RSA, impeding union of these two arteries and originating a left aortic arch with four branches instead of three. The last branch is now the RSA, which is born solitary instead of making part of the BT at the level of the descending aorta and close to the anterior duct, although it has been described in other locations. This origin of the RSA is on the left and, therefore, goes a longer and straight way behind the trachea and the esophagus to reach its final target, the right arm2,4. This abnormality is known as aberrant right subclavian artery or aberrant retroesophageal right subclavian artery or lusoria artery4,5. In most cases, the arterial duct persists on the left side, originating an incomplete vascular ring4. In 80% of cases, the ARSA goes behind the esophagus, in 15% between the esophagus and the trachea, and in 5% of cases in front of the trachea or the main bronchium6 (Figure 1).
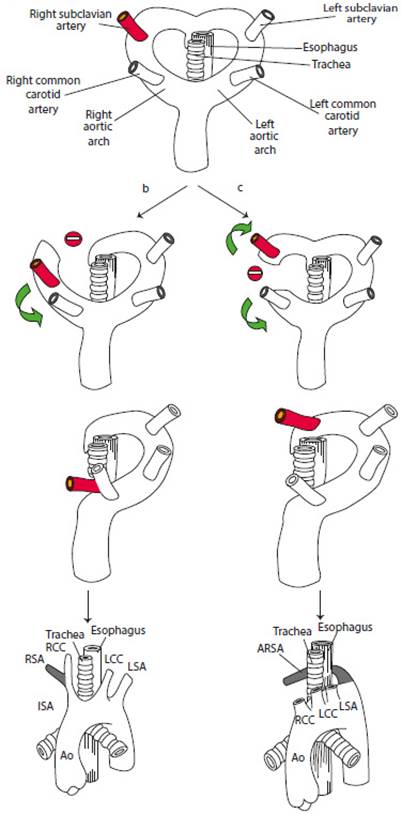
Figure 1 Diagram from Chaoui R, et al. Am J Obstet Gynecol 2005(7) and Chaoui R, et al. Ultrasound Obstet Gynecol 2008(4).
Usually, ARSA is a benign, asymptomatic or oligosymptomatic finding. However, in symptomatic cases there is tracheal compression of either the larynx nerve or the esophagus that results in stridor, difficulty in breathing, recurrent aspirations caused by reflux, and cough or dysphagia in some cases2,3,5.
A normal right subclavian artery is ‘S’-shaped, with anterior concavity in the proximal portion and posterior concavity in the distal portion. It passes in front of the trachea and esophagus and in direction of the right shoulder. If the right subclavian artery is not identified, it is recommended to descend into the axial plane of the three vessels and trachea to evaluate the possibility of an aberrant right subclavian artery. In case of doubt, Doppler color4,7 or pulse-Doppler would confirm the arterial or venous origin of the vessel in question, as the vein shows the typical vein wave2,8-10. It can also be observed in a longitudinal (sagittal) or coronal plane8. The demonstration of the four vessels coming out of the aortic arch in the longitudinal plane is easier to obtain before the 22thweek of pregnancy than in late pregnancies, as the vessels are closer to each other10. Likewise, the coronal plane can be of additional help to find such vascular abnormalities11.
By correctly using the assessment technique and knowing the possible diagnostic errors, success in identifying the normal and aberrant right subclavian artery has an effectiveness of 95% to 98% in the second trimester1,2 and 80% to 85% in the first trimester2,8. These percentages are directly related to the operator experience and indirectly to the maternal body mass index (BMI). In the first trimester, visualization is also directly related to gestational age.
Case report
This 35-year old patient came to our hospital for prenatal control. Her weight before pregnancy was 70 kg, height 156 cm, BMI 28,8. She had regular menstrual cycles, date of her last men struation on May 28, 2019. Current conception was spontaneous. She was a non-smoker and non-drinker.
First trimester analysis was reported normal, toxoplasmosis non-immune, VDRL negative, HIV and hepatitis C negative, hepatitis B immune, measles immune, blood group A Rh +.
Screening for Down syndrome in the first trimester showed low risk 1/7 732.
There was no relevant personal or family medical history for congenital heart disease or sudden death.
Obstetrical history was G3P2, two normal vaginal deliveries in 2015 and 2017 (newborns 3 500 g and 4 000 g, respectively); probable due date March 3, 2020. She presented transient gestational hyperthyroidism; the endocrinologist prescribed no treatment but control 3-4 months postpartum.
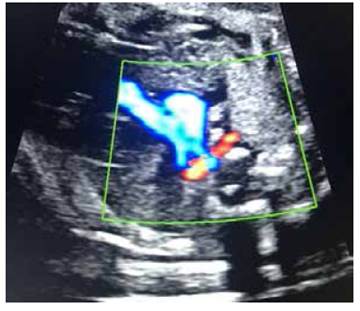
At 20 weeks of pregnancy, fetal anatomy ultrasound showed at the level of the three vessels and trachea, a retrotracheal vessel with suspicion of an aberrant right subclavian artery. No other cardiac or extracardiac structural alteration was identified. Sex was masculine. Figures 2 A and B.
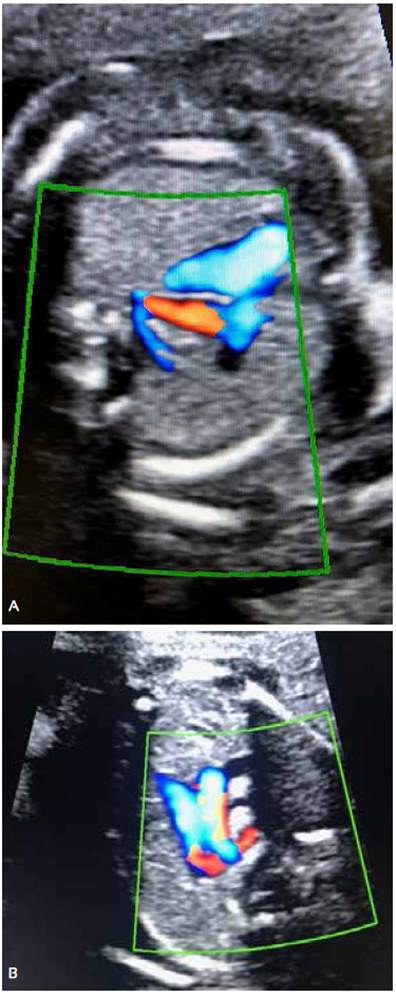
Figures 2 A and B. Ultrasound evaluation at the three-vessel view section using color Doppler shows the corresponding retrotracheal vessel with ARSA.
She was referred to another hospital to confirm diagnosis, and fetal echocardiogram reported situs solitus, levocardia, levoapex, normal segmental arrangement, balanced ventricular volumes with normal transvalvular flows, integral interventricular septum, patent foramen oval, left aortic arch with aberrant right subclavian, left ductus arteriosus and incomplete vascular ring. There was a retrotracheal and retroesophageal vascular structure corresponding to aberrant right subclavian artery. No other extracardiac abnormalities were found. Male sex.
The parents were informed on the ultrasound findings. Association of ARSA with Down syndrome was explained, emphasizing on its low incidence when ARSA appears isolated, almost the same as for normal population. Likewise, they were told about symptoms and how to handle them in the newborn, indicating most patients are asymptomatic. In agreement with the parents, decision was not to conduct invasive tests and continue regular prenatal controls.
At 24 weeks, O’Sullivan test showed normal results. At 28 weeks, the mother received a whooping cough vaccine (protocolized in the Spanish National Health System).
At 33 weeks the fetus showed cephalic presentation by ultrasound, with normal growth, normal levels of amniotic fluid, regular fetal movements. Fetal echocardiogram showed the ARSA and no other cardiac or extracardiac structural pathology. Doppler of the umbilical artery revealed a normal pulsatility index. Figure 3.
At 36 weeks, rectovaginal culture was negative for Streptococcocus agalactie. The patient was referred for fetal monitoring to start at 39 weeks of pregnancy.
The patient was admitted for finalization of pregnancy at 40 weeks and 6 days. Labor was induced using misofar 25 mcg. On March 10, 2020 at 13:50 hours, and under epidural analgesia, the patient presented normal vaginal delivery.
The newborn Apgar score was 9/10 and weight 3 470 g. He was evaluated by the pediatric cardiology service on March 11, 2020. Physical examination showed good general status, normal color, precordial palpation also normal. Femoral pulses were present and symmetric. Cardiac auscultation was rhythmic, with normal first heart sound, no murmur, and normal second heart sound, free diastole. Abdomen within normal limits. Echocardiograph showed normal anatomical relations. Normal lungs. Regular pulmonary and systemic venous drainage. Integral interatrial septum. Normal atrioventricular and sigma valves. Ventricles of regular size and functioning. Ejection fraction 73.5%. No obstruction of outflow tracts.
Normal pulmonary trunk and branches. Left aortic arch with exit of a wide vessel and bifurca tion into two, which impressed as right and left carotids, followed by another vessel of normal diameter, corresponding to the left subclavian artery and, finally, another vessel that was consisting with the aberrant right subclavian, whose course could not be followed. No ductus was observed. Normal abdominal aorta pulsatility. Diagnosis: Aberrant right subclavian artery. Not associated to other cardiac malformations.
Appointment for control was in one month, but in view of the COVID-19 pandemic in Spain, control was by telephone at 19 days of age. The mother did not report noticeable alterations and was called for control by the pediatric service in July 2020, either personal or by telephone, depending on the state of emergency.
Discussion
In most reference centers, evaluation of the right subclavian artery is part of the routine echocardiogram study and is considered a marker of congenital heart disease and Down syndrome1. Finding of an aberrant right subclavian artery should be followed by comprehensive evaluation of fetal and cardiac morphology to rule out other abnormalities. Considering the prevalence on the general population, ARSA finding does not justify a routine invasive examination for Down syndrome2,8. This is backed up by the conclusion of a recent meta-analysis which confirmed that an isolated ARSA acts as a variant of normality9.
The most frequent abnormality of the aortic arch is ARSA, with incidence in the general population and postmortem studies estimated at approximately 0.4% to 2%. Rembouskos et al. prospective study, observed ARSA in 89 of 6 605 fetuses with normal karyotype. Gut et al. investigated the course of the right subclavian artery in 4 125 low-risk pregnancies between 17 and 33 weeks of gestation. ARSA was found in 17 of 4 120 patients (0.4%)3,12. Average prevalence of ARSA in the normal population is 1%13.
The presence of an aberrant right subclavian artery represents 20% higher risk of having another abnormality. Among the associated abnormalities, chromosomal alterations are the most frequent, mainly trisomy 212,3,7,9,10,13.
Identification of ARSA in fetuses with Down syndrome was first described by Chaoui et al. in 200512,13. Prenatal studies report the prevalence of ARSA in 6.8% to 37.5% of fetuses with Down syndrome in the second and third trimesters7,8,12,13, and in up to 18.2% of fetuses with trisomy 186. Paladini et al., with the largest series of patients with Down syndrome, publishes a frequency of 25.5%2.
According to recent publications, the risk for trisomy 21 increases primarily when the finding of ARSA is associated with other markers of triso my 21. When ARSA presents as the only marker of this chromosomal abnormality, risk is not in-creased1,6. Therefore, isolated ARSA may serve as a weak marker for Down syndrome rather than a routine indication for invasive fetal testing13,14. In our case, we informed and explained the parents on ultrasound findings; agreement included non-invasive testing and routine pregnancy monitoring.
Although association between isolated ARSA and chromosome 22q11 microdeletion has not been proven, its detection in conotronchal heart malfunctions is the most important marker of this genetic alteration, so examination is recommended2,8. Offering an invasive procedure to exclude 22q11 microdeletion is under debate. Zidere et al. do not recommend it, unless an extracardiac malformation is present13.
Cardiac abnormalities are the most frequent structural abnormalities that coexist with ARSA (5.4% to 23.5%)12. Most frequent associated cardiopathy is tetralogy of Fallot, which was discussed by Blalock in 19483. Correlation between ARSA and congenital heart disease was reported by Zapata et al, with a postnatal study that included 11 000 cases. They found ARSA incidence was 2.9% in patients with congenital heart disease and 0.1% in patients with normal heart, suggesting relationship between ARSA and con-genital heart disease12. The ratio of ARSA to extracardiac abnormalities has been found to be approximately 5% to 26.7%12.
We conclude that it is possible to evaluate the right subclavian artery in the first and second trimesters of pregnancy, with chances of success of 80% and 90%, respectively. Options for achieving good visualization depend on the operator experience, gestational age and maternal body mass index.
Recommendation when an aberrant right subclavian artery is found is to perform a detailed study of the fetal anatomy in search of other an euploidy markers and a fetal echocardiogram. Invasive studies will be limited to those situations where, in addition to the aberrant right subclavian artery, other markers or conditions that increase the risk of Down syndrome are found.
REFERENCES
1. Touzet GB, Marques Y, Santa Cruz L, Clavelli WA. Diagnóstico prenatal y prevalencia de arteria subclavia derecha aberrante (ARSA) en la evaluación ecográfica morfológica del segundo trimestre. Rev Arg de Ultrasonido. 2017;16(1):28-33. [ Links ]
2. Bravo Arribas C, Gámez Alderete F, Pérez R, Ortiz Quintana L, De León Luis J. Diagnóstico de arteria subclavia aberrante prenatal aislada. Ginecol Obstet Mex. 2012;80(6):425-9. [ Links ]
3. Mijangos Vázquez R, Patiño Bahena E, Martínez García A, Herrera J, Calderón Colmenero J, Buendía Hernández A, et al. Arteria subclavia derecha aberrante en niños examinados en el Instituto Nacional de Cardiología Ignacio Chávez (1992-2002). Arch Cardiol Mex. 2014;84(3):155-61. doi:10.1016/j.acmx.2013.10.010 [ Links ]
4. Chaoui R, Rake A, Heling K. Aortic arch with four vessels: aberrant right subclavian artery. Ultrasound Obstet Gynecol. 2008;31(1):115-7. doi:10.1002/uog.5240 [ Links ]
5. Barriga A, Méndez G. Arteria subclavia derecha retroesofágica: reporte de caso. Int. J. Morphol. 2019;37(3):821-4. [ Links ]
6. Kizilkale O, Torun CY, Yesiladali M, Cenksoy P, Yidirim G, Api O. Importance in prenatal diagnosis of the detection of isolated aberrant right subclavian artery. Perinatal J. 2014;22(1):61-3. doi:10.2399/prn.14.0221011 [ Links ]
7. Chaoui R, Thiel G, Heling KS. Prevalence of an aberrant right subclavian artery (ARSA) in normal fetuses: a new soft marker for trisomy 21 risk assessment. Ultrasound Obstet Gynecol. 2005;26(4):356-356. doi:10.1002/uog.2167 [ Links ]
8. De León Luis J, Gámez F, Bravo C, Tenías JM, Arias A, Pérez R, et al. Second trimester fetal aberrant right subclavian artery: original study, systematic review and meta analysis of performance in detection of Down syndrome. Ultrasound Obstet Gynecol. 2014;44(1):147-53. doi:10.1002/uog.13336 [ Links ]
9. Ranzini AC, Hyman F, Jamaer E, Van Mieghem T. Aberrant right subclavian artery. Correlation between fetal and neonatal abnormalities and abnormal genetic screening or testing. J Ultrasound Med. 2017;36(4):7-90. doi:10.7863/ultra.16.05028 [ Links ]
10. Pavlova E, Atanassova D, Markov D. Isolated aberrant right subclavian artery in third trimester: a case report. Ultrasound Obstet Gynecol. 2013;42(1):121-121. doi:10.1002/uog.12941 [ Links ]
11. Savirón Cornudella R, Lerma Puertas D, Corona Bellostas C, Cotaina Gracia L, Jiménez Montañez L, De León Luis J. Assessment of subclavian arteries: Usefulness of coronal view in prenatal ultrasound diagnosis. J Integr Cardiol. 2017;3(5):1-2. doi:10.15761/JIC.1000227 [ Links ]
12. Song MJ, Han BH, Kim YH, Yoon SY, Lee YM, Jeon HS. Prenatal diagnosis of aberrant right subclavian artery in an unselected population. Ultrasonography. 2017;36(3):278-83. doi:10.14366/usg.16046 [ Links ]
13. Zalei Y, Achiron R, Yagel S, Kivilevitch Z. Fetal aberrant right subclavian artery in normal and Down syndrome fetuses. Ultrasound Obstet Gynecol. 2008;31(1):25-9. doi:10.1002/uog.5230 [ Links ]
14. Sagi-Dain L, Singer A, Josefsberg S, Peleg A, Lev D, Nasser Samra N, et al. Microarray analysis has no additional value in fetal aberrant right subclavian artery: description of 268 pregnancies and systematic literature review. Ultrasound Obstet Gynecol. 2019 Jun;53(6):810-5. doi: 10.1002/uog.20208 [ Links ]
Received: April 16, 2020; Accepted: May 27, 2020











 texto en
texto en