Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.3 Lima jul-sep 2020
http://dx.doi.org/10.31403/rpgo.v66i2267
Case Report
Isolated ventricular septal defect. A case report
1 Gynecology and Obstetrics Service, Hospital de Hellín, Albacete, España
Congenital heart disease is the most common congenital anomaly. Ventricular septal defect (VSD) is a frequent congenital heart disease in newborns, affecting 25 to 30% neonates with cardiac defects. Muscular VSDs are more frequent than perimembranous VSDs. The association of cases with chromosomal anomalies and isolated VSD is relatively low. Spontaneous closure of isolated VSD is higher with small VSD cases, and the muscular VSD is more likely to close spontaneously than the membranous or perimembranous types. Therefore, diagnosis of isolated muscular VSD with no other anomalies can be considered a benign finding.
Key words: Congenital heart defect; Heart septum
Introducción
The ventricular septal defect (VSD) is the most frequent congenital heart disease in newborns, affecting between 25 and 30% of newborns with heart disease1. VSD can be isolated or multiple and is commonly associated with other cardiac defects2. According to systematic studies, the finding of VSD at birth has substantially increased when compared to studies from previous years, possibly caused by a change in detection methods and diagnosis. Recent advances in fetal echocardiography have improved prenatal detection of VSD, especially of the small ones which develop isolated. However, prevalence and distribution of the different kinds of VSD are not well known. Hence, more studies are necessary to determine the postnatal risk of chromosomic anomalies associated to this congenital heart disease when diagnosed prenatally.
Prognosis of an isolated VSD in the postnatal period is good, with great chance of spontaneous closure during the first years of life. However, evolution and outcome of a prenatal isolated VSD have not been established, as only few studies evaluated this type of heart disease when diagnosed during fetal life1.
Extracardiac anomalies associated with VSD include chromosomic anomalies in about 10 to 30% of cases, depending on the defect type and size2. This rate is significantly more elevated than expected postnatally, which certainly has to do with the spectrum of patients diagnosed prenatally with a high proportion of extracardiac malformations associated with VSD. There is also a lack of information on the perinatal evolution of fetal VSD considering spontaneous closure during fetal life or during the first year of life.
Case report
A 35-year-old pregnant woman who started prenatal controls in our hospital, G1 P0 A0, blood type 0 Rh+, suffered of chronic gastritis. With history of tonsillectomy, she did not have drugs and did not smoke. Neither she, her family or her partner had history of heart disease.
First trimester analysis was normal, including hepatitis B, measles, hepatitis C, human immunodeficiency virus, toxoplasmosis and syphilis.
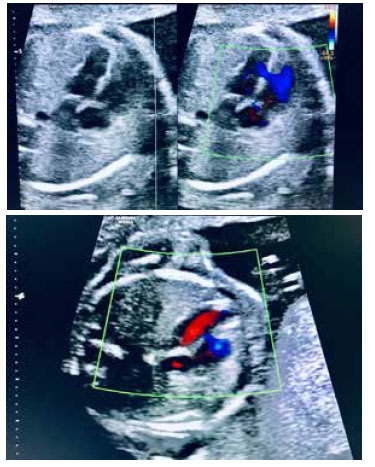
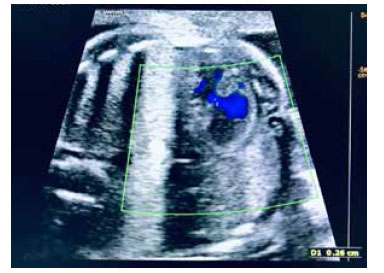
First trimester ultrasound screening showed low risk for Down syndrome 1/7 388. At 20 weeks, an anatomy ultrasound echocardiography showed an isolated ventricular septal defect at a mid-muscular level, with no other associated cardiac or extracardiac anomalies (Figures 1 and 2). Said defect measured was small,2.6 mm, at mid-muscular level (Figure 3). The fetus was male.
In prenatal controls, the ventricular defect was further visualized. At term, the observation was difficulted by fetal position.
The patient was delivered vaginally at 39 weeks gestation. Baby weight at birth was 3 080 g.
Cardiac exploration showed a rhythmic cardiac beat, systolic murmur II/VI, peripheral pulses present and symmetrical. The newborn was transferred to pediatric cardiology at 18 days of life, where the echocardiography showed solid situs, normal segmental ordering, normal atria, interatrial septum with 2 mm patent foramen ovale with left-right-shunt, normal ventricular atrial valves, normal ventricular morphology and disposition, normal contractibility, TAPSE (tricuspid annular plane systolic excursion; indirectly assesses right ventricular function) 13 mm, DVid (left ventricular diastolic diameter) 15 mm, DVis (left ventricular systolic diameter) 10 mm, EF (ejection fraction) 68%. Ventricular septum 4.8 mm with two muscular third interventricular communications (IVC) at the middle third, 2 and 1 mm, with left to right shunt and dynamic gradient 37 mmHg. Normal sigmoid valves. Large sized arteries with normal disposition. Normal venous drainage. Conclusion: Small muscular
IVC without hemodynamic repercussion. Patent foramen ovale (PFO). Control in 6 months.
Discussion
Structural heart abnormalities are estimated to occur in 8-10 out of 1 000 live births at term, and may be 10 times more frequent in premature babies2. Heart abnormalities are often associated with other abnormalities, because the heart begins to develop from the third week following conception and continues to develop until the end of the eighth week. Since the heart basically develops during the entire period of organogenesis, it is susceptible to developing abnormalities3. In addition, many of the cardiac abnormalities are diagnosed in patients without risk factors. Therefore, evaluation of the fetal heart is an important component of routine obstetric ultrasound2,3. The prenatal screening guidelines of the American College of Radiology and of the American Institute of Ultrasonography in Medicine indicate the study of the fetal heart3.
The interventricular septum separates the right and left ventricles and is composed of both muscular and membranous tissues. A normal ventricular septum extends from the cardiac apex to the atrial septum.
The formation of the ventricular septal begins at about 28 days of gestation, when the midline sagittal crest begins to invaginate; the free edge of the muscular septum fuses with the membranous septum formed by endocardial invaginations at 49 days of gestation
A VSD results from poor development of the embryonic muscular septum, poor development of endocardial invaginations, or excess reabsorption of myocardial tissue in the muscular septum3. The diagnosis of fetal VSD can be made by four-chamber cutaway using a side view, to better detect the bidirectional short circuit through the defect. The five-chamber evaluation helps to identify the output defects, primarily membranous VSD output. The objectives of fetal diagnosis are to define the segment of the ventricular septum involved and to exclude another cardiac anomaly2.
VSD is the most common congenital heart disease in newborns4,5, if we exclude the bicuspid aortic valve4. In its isolated form, it represents approximately 20% to 40% of all congenital heart diseases(4,5). Classically, the prevalence was between 1 and 3.5/1 000 live births, and higher in preterm infants. Recently, however, higher numbers have been reported, up to 50/1 000 live births and above. Factors explaining the differences are: the population selected, whether the diagnosis is based on clinical or echocardiographic criteria, and whether or not prenatal diagnoses are included4.
The recent increase of prevalence in newborns is attributed to changes in the diagnostic method and detection modality, including a more frequent use of fetal echocardiography5. Since VSD is an inherited polygenetic condition, it is influenced by genetic factors, environmental factors, or both6. In VSDs not associated with chromosomal disease or with diseases of Mendelian inheritance, the risk of recurrence of congenital heart disease in first-degree relatives of an affected person is between 3 and 4%, with concordance (that is, the heart disease will also be a VSD) in more than half of the cases(4).
VSDs can occur in different locations, including the membranous, muscular, supracrystalline, and atrioventricular inlet valve areas. Muscular VSD can be divided into defects at the muscular half, apical, anterior, and posterior levels. The VSD of the muscular half, the most frequent one, is 5 times more prevalent than the defect at the apical level. The physiological effects of VSD depend on the size of the defect. Although there is no universally accepted definition of VSD size, clinical studies commonly use the following classification: small < 4 mm, medium 4 to 6 mm, and large > 6 mm. Medium and large VSDs can be detected intrauterine as early as 16-18 weeks of gestation; small to moderate VSDs may go undetected during fetal echocardiography(7). In our patient, the VSD corresponded to a medium/ small muscular VSD, evidenced by the 20-week ultrasound and Doppler function.
The detection of VSD before birth has increased exponentially in recent years for several reasons, such as the use of highly qualified equipment, the extensive use of ultrasound in prenatal diagnosis, the training of ultrasound personnel (doctors and technicians), the delay of pregnancy at an older age and the survival of premature babies, all of which increase the prevalence of congenital heart disease and particularly VSD at birth1,7.
The American College of Obstetrics and Gynecology's guide and many other guides to fetal echocardiography propose an anatomical ultrasound scan at 20 weeks of gestation. Prenatal detection of fetal abnormalities improves neonatal survival and reduces morbidity and mortality. This allows for planning, referral and delivery at a tertiary centre, as well as ensuring better perioperative and neonatal care if required. Advanced techniques in fetal echocardiography and their universal distribution have facilitated early detection of even minor congenital heart disease (e.g. small isolated VSDs). In some cases, termination of pregnancy is an option7.
About 50% of people with Down syndrome have congenital heart disease. In a large population study conducted in England from 1985 to 2006, heart abnormalities were identified in 42% of children born with Down syndrome. Although complete atrioventricular septal defect is the most common heart abnormality in Down syndrome, VSDs account for approximately 31% of heart diseases in this group of people. Therefore, further testing to identify the risk of Down syndrome in patients with isolated VSD is part of the debate. Previous studies on the association between VSD and various types of aneuploidy did not distinguish between isolated VSD or VSD associated with other heart abnormalities and major extracardiac abnormalities. Ori Shen et al. reported 92 cases with isolated VSD, and none had trisomy 21. This study is verified by two more, in which no trisomy 21 was found among the 25 to 248 cases of isolated VSD 7. According to these results, trisomy 21 is considered rare when a VSD is the only finding of sonographic abnormality7,8.
The risk of chromosomal abnormality associated with isolated VSD is controversial. According to two large studies, the presence of VSD increases the risk of aneuploidy and extracardiac abnormalities in affected fetuses. However, the course and prognosis of isolated VSD detected prenatally has not been definitively established. Gomez et al. evaluated the risk of chromosomal abnormality associated with prenatal diagnosis of isolated VSD. During the 6-year study period, 248 cases of isolated VSD were diagnosed among 995 cases of congenital heart disease. Amniocentesis for genetic study was performed in 119 pregnancies, and the karyotype of the remaining 129 cases was obtained postnatally. The prevalence of chromosomal abnormality was 1.2% (3/248). In another 5-year study, 534 congenital heart diseases were detected in 23 500 pregnancies. In addition, 76 isolated muscle VSDs were found, but none had a chromosomal abnormality, suggesting that isolated muscle VSDs are benign findings during pregnancy7.
It is known that VSDs can close spontaneously, but the incidence and the mechanism remain unclear. The incidence of spontaneous VSD closure is between 5 and 84%, depending on the size, location and type of defect, as well as the follow-up time. Many studies report that spontaneous closure of VSD occurs more frequently in small defects than in large ones7. It is reported that defects, particularly muscular defects with a diameter < 3 mm, close spontaneously in about 83.8% of cases during pregnancy or during the first year of life7,9.
The incidence of spontaneous VSD closure also varies depending on the site of the defect. Miyake et al. observed that medial trabecular muscular VSD tends to spontaneously close early and more often than anterior and apical trabecular VSD, with a closure range of 83%, 84% and 89% for anterior, apical and medial VSD, respectively.
Spontaneous closure of the VSD also depends on the type of defect. Muscular VSD closes spontaneously more often than the membranous or perimembranous types9.
Spontaneous closure is also related to follow-up time; 32.7% of defects close spontaneously during intrauterine life, 44.3% in the first year of life and 23% remain permeable7,9.
The prognosis of newborns with VSD is related to the location of the defect, its size, and whether or not it is associated with other congenital anomalies7.
It is generally accepted that the prognosis of isolated VSDs in the postnatal period is good, with a high rate of spontaneous closure during the first year of life, which will depend on fetal weight, size of the short circuit measured prenatally and its location10. Isolated muscular VSD is 7 times more prevalent than the perimembranous type and is associated with good prognosis, no cases related to chromosomal abnormalities, risk similar to normal pregnancies11 and 1 to 2% risk of requiring surgery during the first year of postnatal life. Meanwhile, perimembranous VSD is associated with 3.1% risk of chromosomal abnormality; and if the VSD/aorta ratio is above 0.5, the probability of requiring surgery in the first year of life is about 50%. These data can help counseling patients when isolated VSD is detected10,11. In our patient, we agreed with the parents and opted to continue gestation without invasive testing, since we were dealing with an isolated VSD without any other associated cardiac or extracardiac anomaly.
In conclusion, VSD is the most frequent congenital heart disease, and the mid-muscular type is more common than the perimembranous type.
Perimembranous VSDs are associated with high risk of chromosomal abnormalities when compared to muscular VSDs, which have a similar risk to normal pregnancies. The incidence of chromosomal defects and isolated VSDs is relatively low. Spontaneous closure of VSDs depends on the size, site, and type of the defect. The incidence of spontaneous closure of isolated VSD is high for small ones, and the average muscular VSD is the one that most often closes spontaneously compared to the membranous or perimembranous types. Isolated VSD may close spontaneously in utero or postnatally, and the vast majority of mid-muscular VSDs close before the first year of life. In addition, the diagnosis of isolated muscular VSD and no other abnormality can be considered a benign finding.
REFERENCES
1. Gómez O, Martínez JM, Olivella A, Bennasar M, Crispi F, Masoller N, et al. Isolated ventricular septal defects in the era of advanced fetal echocardiography: risk of chromosomal anomalies and spontaneous closure rate from diagnosis to age of 1 year. Ultrasound Obstet Gynecol. 2014;43(1):65-71. Doi:10.1002/uog.12527 [ Links ]
2. Bravo-Valenzuela NJ, Peixoto AB, Araujo Júnior E. Prenatal diagnosis of congenital heart disease: A review of current knowledge. Indian Heart J. 2018;70(1):150-64. doi:10.1016/j.ihj.2017.12.005 [ Links ]
3. Barboza JM, Dajani NK, Glenn LG, Angtuaco TL. Prenatal diagnosis of congenital cardiac anomalies: A practical approach using two basic views. Radiographics. 2002:22(5):1125-38. doi: https://doi.org/10.1148/radiographics.22.5.g02se171125 [ Links ]
4. Malo Concepción P. Insa Albert B. Comunicación interventricular. En: Albert Brotons DC. Cardiología pediátrica y cardiopatías congénitas del niño y adolescente. 3. España: Grupo CTO. 2015:237-53. [ Links ]
5. Erol O, Sevket O, Keskin S, Yazicioglu HF, Gül A. Natural history of prenatal isolated muscular ventricular septal defects. J Turk Ger Gynecol Assoc. 2014;15(2):96-9. doi:10.5152/jtgga.2014.0012 [ Links ]
6. Mu K, Chen L, Wen J, Liu Y, Liu N, Cao DH. Prenatal diagnosis of ventricular septal defect and trisomy 7q11.23q21.3 in two fetuses: A case report. Genet Mol Res 17 (1): gmr16039878. DOI: http://dx.doi.org/10.4238/gmr16039878 [ Links ]
7. Huang SY, Chao AS, Kao CC, Lin CH, Hsieh CC. The outcome of prenatally diagnosed isolated fetal ventricular septal defect. J Med Ultrasound. 2017;25(2):71-5. doi:10.1016/j.jmu.2017.05.005 [ Links ]
8. Shen O, Lieberman S, Farber B, Terner D, Lahad A, Levy-Lahad E. Prenatal isolated ventricular septal defect may not be associated with trisomy 21. J Clin Med. 2014;3(2):432-9. doi:10.3390/jcm3020432 [ Links ]
9. Jin Y, Wang A, Wang Y, Wang Y, Wang W, Hou X. Natural history of prenatal ventricular septal defects and their association with foetal echocardiographic features. Cardiol Young. 2012 Jun;22(3):323-6. doi: 10.1017/S1047951111001521 [ Links ]
10. Li X, Song GX, Wu LJ, Chen YM, Fan Y, Wu Y, et al. Prediction of spontaneous closure of isolated ventricular septal defects in utero and postnatal life. BMC Pediatr. 2016;16(1):207. doi:10.1186/s12887-016-0735-2 [ Links ]
11. Brenner M, Eichhorn KH, Schleubner E. The importance of isolated muscular ventricular septal defect (VSD) - diagnosed in the second trimester - for pregnancy and delivery. Ultraschall Med. 2016;37-SL15_1. Doi:10.1055/s-0036-1587780 [ Links ]
Received: February 11, 2020; Accepted: April 04, 2020











 texto en
texto en