Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.3 Lima Jul-Sep 2020
http://dx.doi.org/10.31403/rpgo.v66i2269
Case Report
Pseudo-Meigs syndrome due to granulosa cell tumor
1 Doctor in Medicine and General Surgery, Hospital de Especialidades San Felipe, Tegucigalpa, Honduras
Meigs’ syndrome is defined as the triad of benign ovarian tumor, pleural effusion and ascites, a rare clinical condition that is treated with tumor resection. Same characteristics may occur in cases of malignant tumors, that add a notable increase in antigen CA-125 serum levels, constituting the pseudo-Meigs syndrome. They have been known for many years, but their pathophysiology remains unclear. We report the case of a pseudo-Meigs syndrome, and a brief bibliography review of the most important characteristics of these syndromes is performed.
Key words: Meigs' Syndrome; Granulosa cell tumor; CA-125 antigen
Introduction
In 1934, Salmon described the association between pleural effusion and benign pelvic tumors for the first time1. In 1937, Joe Vincent Meigs started case descriptions for his book ‘Tumors of the Female Pelvic Organs’, in which some types of ovarian tumors were associated to ascites and pleural effusion. This union of clinical signs has since been known as Meigs’ Syndrome2.
Meigs’ Syndrome consists of an association of a benign ovarian tumor (fibroma, thecoma) with ascites and pleural effusion, in which these manifestations are resolved after the extraction of the tumor3.
Pseudo-Meigs syndrome is defined as the association of ascites and pleural effusion with other benign ovarian tumors (teratomas, cysts, tubal papilloma), malignant tumors (granulosa cell tumor, papillary cystadenoma, Krukenberg tumor, carcinoma, fibrosarcoma) and even uterine leiomyomas2. Although rare, most cases of pseudo-Meigs consist in a conjunction made up by a malignant ovarian tumor, ascites and pleural effusion which does not get resolved with the extraction of the tumor3.
A differential diagnosis must include pseudo-pseudo-Meigs’ syndrome, in which cavity effusions with an elevated concentration of CA-125 are related to systemic lupus erythematosus. Therefore, an elevated concentration of CA-125 can be found in Meigs’, pseudo-Meigs’ and pseudo-pseudo-Meigs’ syndromes; however, this tumoral marker is usually a sign of a malignant ovarian tumor4,5.
The description of a case of pseudo-Meigs’ syndrome caused by a granulosa cell tumor with elevated CA-125 levels follows.
Case report
53-year old patient with a tumor in the right iliac fossa, of 6 months evolution, fast growth, painless, showing an accentuated abdominal perimeter in the last 3 months; and transvaginal bleeding for one-month, in small amounts, redbrown, painless. History of arterial hypertension and diabetes mellitus for the past two years and subclinical hypothyroidism of one-month evolution. History of 6 pregnancies, 4 labors, 2 miscarriages and menopause at age 49.
At first physical examination the patient was stable, had an arterial pressure of 130/90 mmHg, cardiac frequency of 73 bpm, oxygen saturation of 93%, height 1,51 m, 174 cm abdominal perimeter. Ambulation was limited and required mobilization on a wheelchair. Pulmonary auscultation showed diminished vesicular murmur and dull percussion in the right pulmonary base. Abdominal palpation evidenced a positive fluid wave test and a tumor of approximately 20 x 20 cm, well defined, movable, painless, not attached to the dep planes. The gynecologic examination showed hematic rests in the vaginal canal, central, well formed, pink cervix, active bleeding in small amount, without apparent lesions.
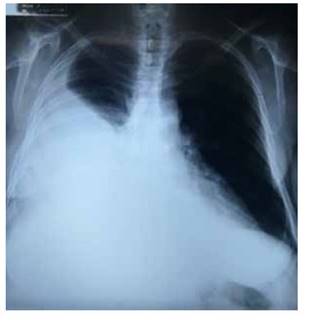
The hemogram results showed normal blood chemistry, coagulation times and thyroid tests. The thoracic X-ray confirmed the right pleural effusion which encompassed approximately 80% (Figure 1). The abdominal tomography informed of a predominantly solid heterogenous mass with liquid areas, the solid part with enhancement to administration of +20 UH contrast medium, size 15 x 15 x 16 cm, in intimate relation with the right adnexa and the uterus, and extending to the mesogastrium. Presence of abundant liquid -more than 2 000 mLin the abdominal cavity. Peritoneal implants could not be ruled out. CA-125 tumor levels were 1 652 U/mL. During hospitalization, the patient showed deterioration of the respiratory function secondary to the cavity spillage, despite several decompressive thoracotomies. A sample sent for study showed leukocyte transudate. The microbiologic cultures were negative and cytology was negative for neoplastic cells.
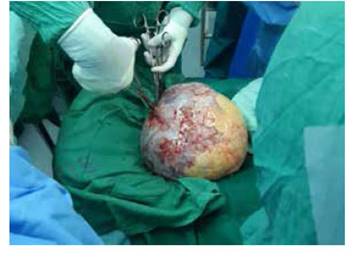
An exploratory laparotomy was conducted with findings of a right ovary giant tumor (Figure 2), solid-cystic, encapsulated, wide cleavage plane, with a large orifice in the capsule that drained abundant serous fluid, probably contributing to ascites. No evidence of peritoneal carcinomatosis was found. 3 500 mL of fluids were removed from the peritoneal cavity. The patient presented sustained hypotension, regardless the adequate fluid management and administration of blood products. Therefore, the surgical procedure was limited to right salpingo-oophorectomy. The ovary pathology report was of a giant granulosa cell tumor size 26 x 24 x 14 cm, 4 200 g, with abundant PAS-positive cells arranged in rosettes (Call-Exner bodies) in all cuts, brown surface with yellowish zones, vascularized; when cut, the cavity was uniloculated, with several solid areas of meaty appearance. On one side, a fragment of the fallopian tube was found with presence of focal necrosis and evidence of infiltration to the capsule, without going through it. Cytology of the peritoneal fluid was negative for neoplastic cells.
Regardless of the suboptimal surgery due to the bad intraoperative status of the patient, the pleural effusion resolved progressively and the ascites did not recur, with evident improvement of the functional state. The patient was referred to the medical oncology service, where she received of cycle of chemotherapy with cisplantin, etoposide and bleomycin.
One year after surgery and 10 months after chemotherapy, the patient is completely recovered. Control abdominal tomography showed no evidence of recurrence or of local or distant metastasis. She will be further followed by the oncology service.
Due to the state of emergency and quarantine at national level due to Covid-19, it was not possible to obtain images of the histological sections.
Discussion
The combination of ascites and elevated levels of CA-125 in a postmenopausal patient increases the suspicion of a malignant ovarian tumor6. The tumor histology confirmed the diagnosis of pseudo-Meigs syndrome. In Meigs’ syndrome, pleural effusion and ascites disappear after removal of the tumor, a phenomenon that does not usually occur in pseudo-Meigs syndrome, because it includes malignant tumors with peritoneal carcinomatosis4. The main tumors related to pseudo-Meigs syndrome are carcinomas, cystadenocarcinomas, granulosa cell tumors and fibrosarcomas7.
Granulosa cell tumors derive from stromal tissues of the sexual cords and are characterized by great hormonal activity, low malignant potential, late recurrences and small rate of distant metastasis. They represent 2 to 6% of ovarian neoplasms and 70% of stromal tumors of the sexual cords5.
After suspicions arise, imaging studies allow a better approximation to definite diagnostic. Tomography is one of the most used techniques.
The radiologic image of the granulosa cell tumor is difficult to differentiate from other adnexal or pelvic tumors, as these vary from solid, heterogeneous or homogeneous to other unilocular or multilocular, with different wall thickness. The granulosa cell tumors usually develop as solid-cystic masses, unilateral, polylobulated, with intratumoral hemorrhagic necrotic regions in central areas, fibroid degeneration, calcifications, septa, papillary structures and a solid, heterogeneous large size appearance, 1 to 30 cm in diameter. This hinders differential diagnosis with cystadenoma, cystadenocarcinomas, other ovarian neoplasms or uterine sarcomas5.
The treatment of choice is complete surgical resection, associated to chemotherapy or adjuvant hormonal therapy, although only systemic therapy appears to have a considerable response, even though partial, and elevated remission rates (50%).
Recurrence rate is 9-35%, mostly during the first 10 years after diagnosis, and low chance of developing distant metastasis (5 to 6%)5.
CA-125 antigen is a glycoprotein with origin in the epithelium of the fallopian tubes, endometrium, pleural mesothelium, peritoneum and pericardium. It is generally associated to malignant ovarian tumors, but can also be elevated in presence of benign tumors8,9. It can also raise during menstruation, pregnancy, endometriosis, peritonitis or liver cirrhosis.
We conclude that etiopathology of cavity spilling is important, but its presence mechanisms are still controversial. Hypotheses include the passage of ascitic fluid through diaphragmatic or lymphatic holes, as well as an increase in capillary permeability caused by the endothelial vascular growth factor - VEGF or proinflammatory cytokines. Pleural transudates could be caused by local changes in hydrostatic or oncotic pressure4.
REFERENCES
1. Liao Q, Hu S. Meigs' syndrome and pseudo-Meigs' syndrome: Report of four cases and literature reviews. J Cancer Therapy. 2015 Apr;6(4):293-8. doi: 10.4236/jct.2015.64032 [ Links ]
2. Barrantes SM. Síndrome de Meigs. Rev Med Sinergia. 2017 Apr;2(4):8-11. [ Links ]
3. Barrantes SM. Síndrome de Meigs y pseudo Meigs: correlación con tumores ováricos. Rev Med Costa Rica Centroam. 2015;71(616):659-62. [ Links ]
4. Modesto-dos Santos V, Correa-Trindade M, Lemes-Duarte M, Manabu-Yano V, Ferreira-Dutra MV, Morais VF, Borges-Viana FGM. Síndrome de Pseudo-Meigs debido al carcinoma seroso de ovario de alto grado. Neumol Cir Torax. 2017 AbrJun;76(2):107-10. [ Links ]
5. Losa EM, Villar M, Pascal A. Síndrome de Meigs y pseudo-Meigs. Clin Invest Gin Obst. 2006;33(1):25-34. doi: https://doi.org/10.1016/S0210-573X(06)74078-3 [ Links ]
6. Saha S, Robertson M. Meigs' and Pseudo-Meigs' syndrome. Australasian J Ultrasound Med. 2012;15(1):29-31. doi:10.1002/j.2205-0140.2012.tb00140.x [ Links ]
7. Sánchez-Torres DA, Díaz-Murillo R, Kazlauskas S, de Santiago J, Zapardiel I. Síndrome de Meigs por fibroma ovárico bilateral parecido al cáncer de ovario. Ginecol Obstet Mex. 2016 feb;84(2):122-5. [ Links ]
8. Quinlan D. Meigs' Syndrome: Image of the month. J Obstet Gynaecol Can. 2012;34(4):311. https://doi.org/10.1016/ S1701-2163(16)35204-5 [ Links ]
9. Virelles-Pacheco A, Guerra-Verdecia C, Batista-Cedeño C, Proenza-Macías J, Santiesteban-Vázquez R. Síndrome de Meigs. Presentación de un caso y revisión de la entidad. Rev Med Multimed [revista en Internet]. 2017; [citado 2020 Jun 20]; 20(5):[aprox. 7 p.]. Disponible en: http://www.revmultimed.sld.cu/index.php/mtm/article/view/402 [ Links ]
Received: February 28, 2020; Accepted: June 19, 2020











 text in
text in