Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.3 Lima jul-sep 2020
http://dx.doi.org/10.31403/rpgo.v66i2264
Case Report
Primary ovarian carcinoid tumor. Case report
1 Endocrinology Service, Hospital Príncipe de Asturias, Alcalá de Henares, España
2 Pathology Service, Hospital Príncipe de Asturias, Alcalá de Henares, España
3 Department of Research and Development, Hospital Central Dr. Urquinaona, Maracaibo, Venezuela
Primary neuroendocrine tumors are rare. They belong to a group of heterogeneous neoplasms that express similar immunohistochemical markers. Carcinoid tumors are the most common neuroendocrine neoplasms. Most of them arise in the gastrointestinal and bronchopulmonary tract. Primary carcinoid tumors of the ovary are rare entities that represent approximately 0.3% of all carcinoid tumors and less than 0.1% of all ovarian neoplasms, with good prognosis and generally limited to the ovarian parenchyma. These tumors arise from the ovarian stromal neuroendocrine cell system, superficial epithelium, and teratomas. In most cases, clinical manifestations are associated with the release of vasoactive substances that cause symptoms such as skin redness, diarrhea, and bronchospasm. For diagnosis it is necessary to use multimodal radiological images and biochemical analysis of neuroendocrine tumor markers. First-line treatment is tumor resection whenever possible. Prognosis is generally favorable, except in some cases with metastases. A case of primary ovarian carcinoid tumor is presented.
Key words: Primary carcinoid tumor; Neuroendocrine tumor; Ovary; Carcinoid
Introducción
Neuroendocrine tumors are epithelial neoplasms with predominant differentiation of neuroendocrine cells, of which carcinoid tumors comprise the main subtype. Primary ovarian carcinoid tumors are rare, accounting for 0.3% of all carcinoid tumors and less than 0.1% of malignant ovarian tumors. They originate most frequently from the gastrointestinal and bronchopulmonary systems. Those appearing in the ovary may be primary or metastatic1.
Most ovarian primary carcinoid tumors are unilateral and classified into four types: insular (the most common), trabecular, mucinous, and mixed2. Neuroendocrine tumors release a large amount of serotonin into the systemic circulation and cause manifestations of carcinoid syndrome in the absence of metastasis3. Its rarity within the genital tract may lead to late diagnosis unless patient's systemic symptoms are recognized for being related to pelvic pathology. Other types are generally not associated with the carcinoid syndrome. A case of a primary ovarian carcinoid tumor is presented.
Clinical case
A 60-year-old female patient consulted because of exertional dyspnea after walking a few meters, lower limb edema and abdominal pain, which progressively increased in the previous six months, accompanied by abrupt facial redness that progressively worsened (around 15 episodes per day), and later diarrhea (four to five episodes per day), anorexia, night sweats, and abdominal distention, for the past five years. She reported a history of chronic arterial hypertension treated with calcium channel blockers. She denied chest pain, palpitations, paroxysmal nocturnal dyspnea, and other significant medical, surgical, or family history.
On physical examination, temperature was 37.7°C, heart rate 100 beats per minute, respiratory rate 16 breaths per minute, and blood pressure 145/95 mmHg. Heart sounds were rhythmic and without murmurs, and vesicular murmur was audible in both lung fields, with no aggregates. The abdomen was globose, with evidence of hepatomegaly, changing dullness, and a positive wave sign, with a palpable, hard and irregular tumor in the suprapubic area.
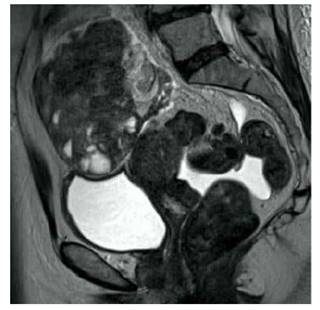
Electrocardiogram revealed sinus rhythm with an incomplete right bundle branch block, and plain chest X-ray showed mild cardiomegaly without signs of pericardial calcifications, with lung fields clear. Abdominopelvic ultrasound revealed the presence of a tumor in the right ovary measuring 11 x 8 x 6 centimeters with dominant peripheral vascularization, arterial and venous waves. Magnetic resonance images revealed a solid-cystic pelvic tumor of approximately 12 centimeters, apparently arising from the right ovary, with a scant amount of free fluid in abdominal cavity (Figure 1). No intestinal, liver, or bladder lesions were observed. The upper and lower endoscopy did not show evidence of gastrointestinal pathologies and chest tomography did not show tumors.
In view of the possibility of a neuroendocrine tumor, the following tests were ordered: urinary 5-hydroxyindolecentic acid (5-HIAA) 65 mg / 24 hours (normal value (NV) 2 to 6 mg / 24 hours), serotonin 1 318 mcg / L (NV 80-400 mcg / L) and chromogranin A 1 130 mcg / L (NV 27 to 94 mcg / L). Gastrin, vasoactive intestinal peptide, glucagon, and calcitonin values were within normal limits. Hematology, renal function, electrolytes, coagulation profile, liver function, and tumor marker values alpha-fetoprotein, human chorionic gonadotropin, carcinoembryonic antigen, and CA-125 were all within normal limits.
Positron emission tomography showed a pelvic tumor with moderate to intense uptake of gallium-labeled octreotide, with no evidence of metastatic intestinal disease, suggesting the possibility of a primary ovarian carcinoid tumor.
Patient underwent laparotomy while being treated with octreotide (50 mcg / h 24 hours before surgery). An ovoid, solid, multinodular, and irregular tumor of approximately 12 centimeters originating from the right ovary, firmly adhered to the cul-de-sac, without apparent infiltration to adjacent structures was found. A frozen biopsy of tumor was performed, and squamous epithelium, mature respiratory epithelium and bone, cartilage, smooth muscle, adipose, and brain tissue (glial and choroidal tissue) were observed.
Given the findings, a total hysterectomy plus bilateral oophorosalpingectomy, peritoneal lavage, omentectomy, pelvic and para-aortic lymphadenectomy were performed. There were no complications during surgery and she was discharged on day four. Chromogranin A (17 mcg / L) and urinary 5-HIAA (2.8 mg / 24 hours) values were within normal limits six weeks after surgery. Symptoms also disappeared. Patient did not return for postoperative follow-up.
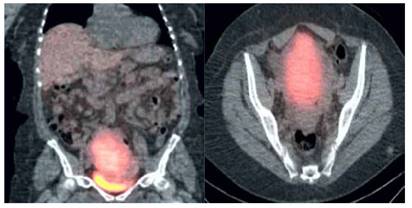
Figure 2 Positron emission tomography image showing pelvic tumor with Moderate to intense gallium-labeled octreotide uptake.
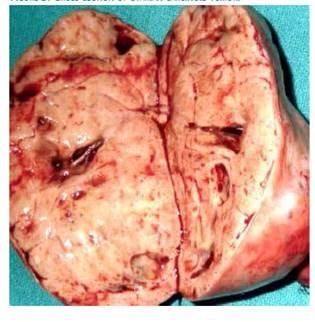
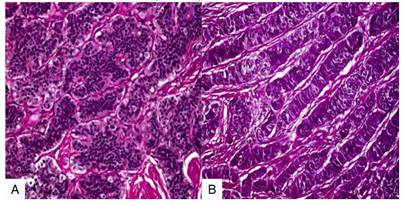
Macroscopic study showed a solid, smooth-surfaced, congestive tumor measuring 9 × 6 × 5 centimeters confined to the right ovary, with intact capsule and weighing 152 grams (Figure 3). On section, a grayish, nodular area was observed, with no evidence of cystic changes. Microscopic evaluation showed uniform polygonal cells with eosinophilic granular cytoplasm and regular nuclei with little mitotic activity, arranged in solid sheets with a trabecular and insular pattern. These patterns were lined with layers of cells with central, homogeneous, and round nuclei with small nucleoli without mitosis or atypia (Figure 4). The amount of cytoplasm in the cells was large and strongly acidophilic. Immunostaining showed strong positivity for CK19 along with diffuse immunostaining for CD56 and localized immunopositivity for CK7, NSE, CDX2, and synaptophysin and negative for CK20, CEA, CA125, TTF-1, HNF1 beta, and MiB with Ki67 dispersed in less than 1% in the minor individual cell staining. Ultrastructural study of the cells revealed numerous intracytoplasmic neurosecretory granules. Final diagnosis was primary ovarian neuroendocrine carcinoid tumor.
Discussion
Carcinoid tumors are well-differentiated neuroendocrine tumors that arise from enterochromaffin cells and secrete serotonin and other vasoactive substances. Most carcinoid tumors are found in the gastrointestinal tract (55%) and bronchopulmonary region (30%). The small intestine is the most common site (45%), followed by the rectum (20%), appendix (17%), colon (11%), and stomach (7%). Primary ovarian tumors are even rarer, accounting to less than 0.1% of ovarian malignancies and 1% of all carcinoid tumors4.
Most carcinoid tumors are relatively slow-growing, but they often metastasize and some can behave aggressively. Approximately 19% of carcinoid tumors present with metastatic disease; the most common site is the liver, regardless of its primary origin. Even with significant hepatomegaly caused by infiltration of the liver parenchyma due to metastatic lesions, liver biochemistry may be within normal limits5. Ovarian involvement can be primary or metastatic nature. Primary ovarian carcinoid tumors are generally unilateral and are composed of epithelial elements of gastrointestinal or respiratory origin. They often arise within a cystic teratoma or dermoid tumor, and up to 60% coexist with these tumors6.
Primary ovarian carcinoid tumors are accidentally discovered in most women by ultrasound imaging7. Patients rarely present with abdominal pain, constipation, hirsutism, or a pelvic mass. Clinical entity is probably multifactorial and mediated by vasoactive hormones, such as serotonin, tachykinins, kallikrein, and prostaglandins. All these substances are metabolized and inactivated by hepatic monoamine oxidases. However, in some cases, presence of liver metastases is not required for the development of the syndrome, since these substances can reach systemic circulation directly when the primary tumor is in the lung or the ovary. In the latter case, vasoactive substances reach the systemic circulation through the inferior vena cava (right ovary) or renal vein (left ovary)8.
Diagnosis of carcinoid tumors requires multiple imaging modalities and biochemical tests if there are symptoms of carcinoid syndrome. Computed tomography (76% sensitivity, 96% specificity) and ultrasound (93% sensitivity, 95% specificity) are useful for locating the tumor, although they cannot identify whether the lesion is carcinoid9. Functional imaging or photon emission tomography can confirm diagnosis, identify metastases, and stage of disease. This requires administration of radiolabeled octreotide, a synthetic analog of somatostatin that is absorbed by carcinoid tumors. These tests have a sensitivity of 94%10.
Those with symptoms suggestive of carcinoid syndrome may have elevated 24-hour urine 5-HIAA (the main breakdown product of serotonin) as a screening test. Elevated urinary concentrations confirm presence of the syndrome and guide the use of imaging to locate and stage the tumor. Chromogranin A can also be used as a marker, but its specificity is lower because it is also secreted by pheochromocytomas10.
Carcinoid tumors are characterized by growth patterns that suggest neuroendocrine differentiation, including organoid, insular, trabecular, palisade, ribbon, and rosette arrangements. Individual tumor cells have uniform cytologic features with moderate, finely granular eosinophilic cytoplasm and nuclei with a finely granular chromatic pattern. Histological criteria to discriminate between typical and atypical ovarian carcinoid tumors have not been established. Since ovarian carcinoid incidence is very low, with no cases of aggressive histological characteristics reported, there was no prior need to establish criteria for atypical carcinoid tumor of the ovary11. Primary ovarian carcinoid tumors composed of more than one pattern may be classified as primary mixed carcinoid tumors, regardless of the predominant pattern12. Trabecular carcinoid tumors neurosecretory granules are round to oval and show slight variations in size, thus differing from marked variations seen in insular carcinoid tumors. Immunocytochemical analyses of trabecular carcinoid tumors show a much broader range of neurohormonal polypeptides compared to those in insular carcinoid tumors13.
Primary ovarian carcinoid tumors must be differentiated from metastatic carcinoid tumors or cord-like pattern Sertoli-Leydig cell tumors. Metastatic carcinoid tumors are bilateral, with peritoneal metastases and poor prognoses; presence of teratomatous elements help to exclude a metastatic lesion13. The most common subtypes that metastasize to the ovary are insular and trabecular7. Sertoli-Leydig cell tumor shows similar cordon patterns and, unlike trabecular carcinoids, has inhibin-positive tubular formations and absence of neurosecretory granules13.
Therapeutic options are complex. Treatment with somatostatin analogs accompanied or not by tumor reduction-resection may improve symptoms and the negative hemodynamic changes on cardiac function8. Somatostatin analogs inhibit the release of biogenic amines and peptides, including serotonin, and relieve symptoms. Surgery is the standard treatment for resectable tumors, although it is not often possible in patients with metastatic disease. Cytoreductive surgery and chemotherapy are often necessary to prolong survival. Octreotide is useful in patients with symptoms of carcinoid syndrome to relieve symptoms and prevent progression of heart disease14. Perioperative treatment should include intravenous infusion with octreotide to reduce the risk of carcinoid crisis. Antihistamines and corticosteroids may be helpful in controlling flushing and bronchospasm. There is no evidence to support the use of adjuvant treatments (hormones, chemotherapy, or radiation)8.
Prognosis is excellent for stage I ovarian carcinoid tumors with 5-year survival rate of 90%. Metastatic carcinoid tumors (most commonly of small intestine) tend to be bilateral, insular type, with tumor deposits in both ovaries. In this group of patients, prognosis is poor since survival rate is less than 50% at five years. The 3-year mortality rate for patients with carcinoid syndrome and heart disease is 31%, while those without heart disease have approximately twice the survival rate15.
In conclusion, primary ovarian carcinoid tumor is a very rare neuroendocrine neoplasm, which should be considered in patients with carcinoid syndrome symptoms with location other than the gastrointestinal tract or lungs. Patients with these ovarian tumors and systemic symptoms must be evaluated for endocrinological-cardiac status and undergo early surgery, to avoid progression to cardiac syndrome. Symptoms should be considered to facilitate early diagnosis and treatment of this curable malignancy, with excellent survival rates.
REFERENCES
1. Chiu HC, Chen YL. Trabecular carcinoid tumor arising from a mature cystic teratoma. Ci Ji Yi Xue Za Zhi. 2019;31(3):192-4. doi: 10.4103/tcmj.tcmj_91_18 [ Links ]
2. Saraf K, Tingi E, Brodison A, Sinha S. A rare case of primary ovarian carcinoid. Gynecol Endocrinol. 2017;33(10):766-9. doi: 10.1080/09513590.2017.1328049 [ Links ]
3. Hsu WW, Mao TL, Chen CH. Primary ovarian mucinous carcinoid tumor: A case report and review of literature. Taiwan J Obstet Gynecol. 2019;58(4):570-3. doi: 10.1016/j.tjog.2019.05.025 [ Links ]
4. Detterbeck FC. Management of carcinoid tumors. Ann Thorac Surg. 2010;89(3):998-1005. doi: 10.1016/j.athoracsur.2009.07.097 [ Links ]
5. Oberg K, Couvelard A, Delle Fave G, Gross D, Grossman A, Jensen RT, et al. ENETS Consensus guidelines for standard of care in neuroendocrine tumours: Biochemical markers. Neuroendocrinology. 2017;105(3):201-11. doi: 10.1159/000472254 [ Links ]
6. Shehabeldin AN, Ro JY. Neuroendocrine tumors of genitourinary tract: Recent advances. Ann Diagn Pathol. 2019;42:4858. doi: 10.1016/j.anndiagpath.2019.06.009 [ Links ]
7. Rouzbahman M, Clarke B. Neuroendocrine tumors of the gynecologic tract: select topics. Semin Diagn Pathol. 2013;30(3):224-33. doi: 10.1053/j.semdp.2013.06.007 [ Links ]
8. Lagoudianakis EE, Markogiannakis H, Karantzikos G, Papadima A, Alevizos L, Manouras A. Primary insular carcinoid of the ovary. Eur J Gynaecol Oncol. 2008;29(5):554-5. [ Links ]
9. Kanakis G, Kaltsas G. Biochemical markers for gastroenteropancreatic neuroendocrine tumours (GEP-NETs). Best Pract Res Clin Gastroenterol. 2012;26(6):791-802. doi: 10.1016/j.bpg.2012.12.006 [ Links ]
10. Rubin de Celis Ferrari AC, Glasberg J, Riechelmann RP. Carcinoid syndrome: update on the pathophysiology and treatment. Clinics (Sao Paulo). 2018;73(suppl 1):e490s. doi: 10.6061/clinics/2018/e490s [ Links ]
11. Min KW. Two different types of carcinoid tumors of the lung: immunohistochemical and ultrastructural investigation and their histogenetic consideration. Ultrastruct Pathol. 2013;37(1):23-35. doi: 10.3109/01913123.2012.707962 [ Links ]
12. Metwally IH, Elalfy AF, Awny S, Elzahaby IA, Abdelghani RM. Primary ovarian carcinoid: A report of two cases and a decade registry. J Egypt Natl Canc Inst. 2016;28(4):267-75. doi: 10.1016/j.jnci.2016.06.003 [ Links ]
13. Euscher ED. Germ cell tumors of the female genital tract. Surg Pathol Clin. 2019;12(2):621-49. doi: 10.1016/j. path.2019.01.005 [ Links ]
14. Korda D, Doros A, Piros L, Gerlei Z, Haboub-Sandil A, Mándli T, et al. Liver transplant for metastatic neuroendocrine tumors: A single-center experience in Hungary. Transplant Proc. 2019;51(4):1251-3. doi: 10.1016/j.transproceed.2019.04.010 [ Links ]
15. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335-42. doi: 10.1001/jamaoncol.2017.0589 [ Links ]
8Ethical responsibilities: Protection of human subjects: The authors declare that the procedures performed were in accordance with the ethical provisions of the corresponding human research ethics committee, as well as the World Medical Association and the Declaration of Helsinki.
9Data confidentiality: Authors declare that they have followed the protocols issued by the Hospital Central "Dr. Urquinaona" regarding publication of patient’ data.
10Right to privacy and informed consent: The authors obtained informed consent from all patients and/or subjects present in the paper. This corresponding author is in possession of these documents.
Financing: The authors certify that they have not received financial support, equipment, work personnel nor objects from people, public and/or private institutions to perform this study.
Received: September 11, 2019; Accepted: January 26, 2020











 texto en
texto en