Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Peruana de Ginecología y Obstetricia
versão On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.4 Lima out-dez 2020
http://dx.doi.org/10.31403/rpgo.v66i2287
Special Articles
Management of colorectal cancer during pregnancy
1. Doctor in Clinical Medicine, Specialist in Gynecology and Obstetrics, Obstetrics and Gynecology Service, Central Hospital "Dr. Urquinaona , Maracaibo, Zulia State, Venezuela
Cancer during pregnancy has increased due to delayed motherhood and the known occurrence of age-dependent malignant tumors. With the increase in colorectal cancer diagnosed in young adults, the number of cases during pregnancy is likely to increase. Colorectal cancer is one of the 3 most common types of cancer in women, but the occurrence during pregnancy is rare and is associated with a large number of diagnostic and therapeutic challenges since the diagnosis is usually made in advanced stages. Besides, early diagnosis is difficult because the signs/symptoms (vomiting, constipation, anemia, rectorrhagia, pain and bloating) can simulate those observed during pregnancy. After diagnosis, the therapeutic plan must be established immediately, according to gestational age, by a multidisciplinary medical team. Doctors who care for pregnant women with colorectal cancer face two problems: the need for early maternal cancer treatment and timing of pregnancy termination. Professionals must become familiar with the challenges associated with systemic treatment, since antineoplastic treatment presents several unknowns for its use.
Key words: Colorectal neoplasms; Pregnancy; Pregnancy complications; neoplastic
Introduction
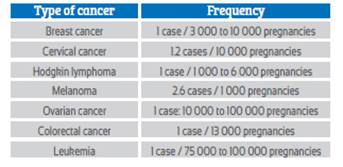
Colorectal cancer (CC) is the second cause of morbidity and third cause of mortality from malignant neoplasms in women. In Peru, it is the sixth leading cause of cancer mortality1. About 10% of cases affect women under 40 years of age and only 2% under 30 years2-4. Appearance of malignant tumors in pregnant women is a rare phenomenon and one of the rarest neoplasms is CC (Table 1). The incidence in pregnant women varies between 0.002% and 0.007%2,3. Different studies show that risk of suffering from it during pregnancy is 0.028 cases per 1 000 births4.
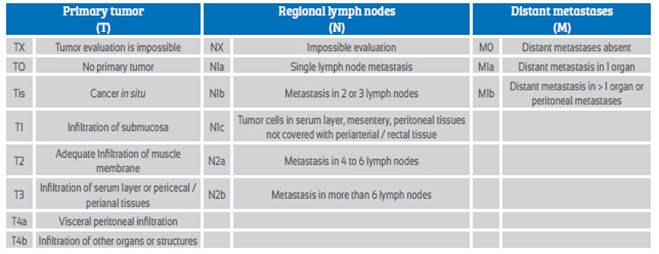
Due to the low frequency of CC in pregnant women, there are no diagnostic and therapeutic schemes supported by clinical trials. Current knowledge is based primarily on case reports. Treatment is determined by clinical stage of disease and gestational age of pregnancy at the time of diagnosis. The TNM classification is the most widely used in clinical practice and allows a precise determination of the severity of condition (Table 2). The objective of this review is to establish the management of colorectal cancer during pregnancy.
Methodology of the infomation search
Between July and December 2019, electronic databases of biomedical scientific literature (UP To DATE, OVIDSP, ScienceDirect, SciELO and PUBMED) were examined to investigate eligible articles in the last 30 years (1989 to 2019). The search terms were: "Colorectal cancer", "Pregnancy", "Colorectal malignant neoplasms", "Diagnosis", and "treatment". Articles in English and Spanish of studies carried out in humans were included, subsequently carrying out an analysis of the different aspects of diagnosis, treatment, and management of CC in pregnant women.
Diagnosis
Changing bowel habits, constipation, or rectal bleeding are common symptoms of CC. However, the appearance of these manifestations during pregnancy does not lead to diagnostic suspicion of CC and they are often underestimated. This can lead to late diagnosis and treatment.
The simplest, cheapest and most accessible clinical evaluation is digital rectal examination. It often allows the tumor to be diagnosed during first visit. All patients with symptoms, especially when they appear before pregnancy and / or intensify despite treatment, should undergo a rectal examination, even more so if they refer to a history of rectal or anal pathology. The most common reasons that do not allow rectal exam are when it is considered that the symptoms are related to pregnancy, such as hemorrhoids, and patient's reluctance.
In case of suspected CC in pregnant women, it is advisable to perform a colonoscopy. This method is safe during pregnancy, especially during second and third trimesters5. Endoscopic evaluation should not be delayed, especially if symptoms persist and are not related to pregnancy. An additional advantage is the ability to sample suspicious sites and make a reliable histopathological diagnosis. Most tumors in pregnant women appear in rectum or sigmoid colon (60% to 80%)6. Therefore, sigmoidoscopy may be sufficient for diagnosis, shortening examination time and reducing risk of complications. Colonoscopy during the first trimester of pregnancy is associated with increased risk of miscarriage. Other adverse effects include: teratogenic effects of anesthesia, increased risk of placental insufficiency, hypoxia, hypotension and premature detachment of the placenta5,7. To reduce these risks, it is advisable to use meperidine (instead of benzodiazepines), an oxygen mask, gentle abdominal pressure, and continuous fetal monitoring.
If imaging is necessary, methods of choice are ultrasound and magnetic resonance imaging8.
However, visualization of colorectal tumors by ultrasound is difficult. However, sensitivity to detect liver metastases is 75%9. Magnetic resonance imaging is most useful to accurately detect pathological changes in bowel and liver. Other diagnostic imaging methods, such as plain abdominal radiography and computed tomography, have an increased risk of fetal complications, mainly due to ionizing radiation, a known teratogenic factor10.
Fetal effects of ionizing radiation depend on dose, location of irradiated site, and gestational age11,12. There is evidence showing that gestational age is not associated with fetal radiation absorption. Estimated fetal dose for abdominal radiographs in two views is 0.02 rads. Abdominopelvic computed tomography produces a fetal irradiation dose of 2.6 rads13. Several studies have shown that a fetal radiation dose greater than 5 rads does not produce adverse effects, especially after organogenesis11,12,14. A dose of 10 to 20 rads causes malformation and lowers intelligence quotient in newborns11. Fetal exposure to doses less than 1 rad increases risk of childhood neoplasms (especially leukemia) from 2 to 4 cases per 1 000 pregnancies11,12.
In some situations, it is necessary to use diagnostic methods that use radiation. For example, acute intestinal obstruction requires quick and accurate diagnosis. This occurs secondary to CC in 7% to 30% of the general population15. However, during pregnancy, it is extremely rare and there are only 2 cases described16,17.
In pregnant women, serum determination of tumor markers has limitations. Concentrations of CA-125, human chorionic gonadotropin, alpha-fetoprotein, and protein 4 of the human epididymis are variable during pregnancy and cannot be considered reliable indicators of tumor process nor useful in routine clinical practice18. On the other hand, concentrations of carcinoembryonic antigen, CA 19-9 and lactate dehydrogenase do not show significant changes during pregnancy19. Serum carcinoembryonic antigen concentrations may be useful in cases of CC. However, they have too low sensitivity and specificity to be used routinely in the diagnosis of CC. And, in pregnancy, although it is one of the first tests performed, it is not very useful20, especially since the increase in concentrations is proportional to the CC stage, and 60% of cases in pregnant women are diagnosed in Dukes C stage6. However, it can be useful for evaluating therapeutic effectiveness and monitoring for recurrences. Neither can it be used to complete diagnosis, especially in cases where alarm symptoms persist.
Treatment
Choosing appropriate therapeutic strategy is a challenge for physicians (Figure 1). Decision on method, technique, treatment time and delivery method must be considered individually for each case by a multidisciplinary team. The ethical problem is to establish the moment of termination of the pregnancy, the chances of fetal survival, the risk of disease progression, and the prognosis. Therefore, gestational age, maternal age, number of children, and stage of cancer must always be taken into account.
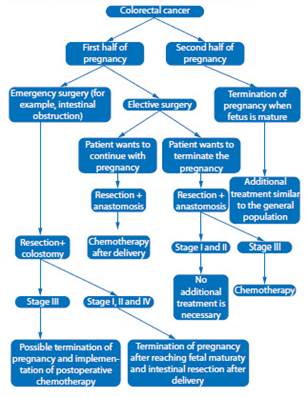
Figure 1 Diagram of therapeutic management of colorectal cancer during pregnancy (adapted from Salani et al., 201419).
Although there is no evidence that CC can have direct fetal effects, even in advanced stages, it is clear that fetal development depends on maternal well-being21. During evolution of cancer, anemia, hypoproteinemia and malnutrition can be found, which can interfere with fetal growth.
If CC is diagnosed during the first half of pregnancy (less than 20 weeks' gestation), all therapeutic options should be considered, including termination of pregnancy22. Due to the small number of cases in pregnancies less than 20 weeks, there are limited data on optimal management23. In those women who do not wish to interrupt the pregnancy, it is advisable to postpone surgery until the second trimester, since performing it in the first trimester is associated with a higher risk of miscarriage24.
Recommended procedure in pregnant women is tumor resection. There is evidence that Miles surgery can be performed in the first trimester without manipulation of the pregnant uterus9. Low anterior resection is the preferred method, due to fewer side effects and better quality of life25. In the case of elective surgery, intestinal anastomosis is recommended. Hartmann surgery should be reserved for emergencies.
In those cases of CC detected after 20 weeks, treatment should be postponed until postpartum, unless there are urgent indications26. The time for termination of pregnancy is between the 32nd and 35th week27, but it can be planned before this period9. In cases of interruption before 34 weeks, corticosteroids should be administered to accelerate fetal lung maturation. If there are indications for cesarean section or the tumor size prevents delivery, it is possible to perform a cesarean section together with intestinal resection. In cases of vaginal delivery, surgery should be postponed until 6 weeks after delivery, when uterine size and vascularity decrease, increasing the possibility of better results. Regardless of route of delivery, the placenta should always be subjected to histopathological examination, due to the possibility of metastasis28.
For acute intestinal obstruction, colonoscopic stents may be placed. This allows stabilization of the patient, preparation and planning of surgery, as well as administration of steroids to stimulate fetal lung maturation16,17.
CC with metastases to ovaries with high concentrations of carcinoembryonic antigen is an unfavorable prognostic factor29-31. Prevalence in general population is 3% to 8%, but in women under 40 years of age it increases to 23%6,31. In pregnant women, ovarian metastasis is present in 25% of cases21,30,32. There is no agreement as to whether appendectomy is necessary during the initial surgery. Some authors recommend bilateral adnexectomy, as it reduces risk of disease progression and protects against primary ovarian cancer. However, this therapeutic approach increases the risk of miscarriage, especially during the first trimester33. Removal of adnexa and cecal appendix prolongs survival by 10 months34. Other authors have not been able to confirm these findings35.
Chemotherapy is widely used in the treatment of CC. However, almost all cytotoxic drugs cross the placental barrier and fall into category D36. The effect on the fetus / neonate depends on the drug, dose, time and duration of exposure. In addition, multi-drug treatment increases the risk of side effects (25%) compared to monotherapy (17%)37. The greatest fetal risk is associated with alkylating agents and antimetabolites38. The safest are platinum derivatives, and taxanes39. The unfavorable fetal effect is more marked during the first trimester, and especially during organogenesis40. The risk of adverse outcome in the first trimester of pregnancy is 10% to 20%, compared with 3% in healthy pregnant women41. After organogenesis, the most vulnerable organs and systems are the reproductive, central nervous, bone marrow and ocular.
Although there are no doubts about the fetal effects of chemotherapy during the first trimester, the effect of cytostatics after this period is unclear. The risk of birth defects in the second and third trimesters is 8% and 6%, respectively39. Chemotherapy after 13 weeks increases the risk of intrauterine growth restriction42. Several authors contradict these findings, showing that chemotherapy in this period does not increase the risk of birth defects, preterm delivery, or intrauterine growth restriction43. Different studies have not shown effects of the chemotherapy after the first trimester, on growth, development or any type of hematological and immunological abnormalities44.
The basic drug for the treatment of CC is 5-fluorouracil, which belongs to the antimetabolites by interrupting DNA synthesis, and is independent of the cell cycle. In animal studies it produces fetal death and in humans it is dangerous during the first trimester of pregnancy. There are reports of fetal congenital malformations in 14% to 19% of cases. After 13 weeks, the birth defect rate de-creases to 1.3%45. Oxaliplatin, a third-generation platinum derivative, damages and blocks DNA and RNA synthesis. In animals it causes spontaneous abortions and fetal intrauterine growth restriction. Adverse effects of chemotherapeutics on pregnancy have only been confirmed in animal studies. Therefore, the simple transfer of these results to humans can be unreliable46. The most common chemotherapy regimens used in pregnant women with CC is the combination of folinic acid, 5-fluorouracil and oxaliplatin or 5-florouracil and leucovorin. Only one neonate with endocrine and neurological alterations has been reported47.
The use of radiotherapy in the treatment of CC in pregnant women is limited. Doses greater than 20 rads are teratogenic and cause spontaneous abortion38. Most treatment regimens propose alternative treatment methods (for example, preoperative chemotherapy) and recommend postponing radiotherapy until after delivery47.
An additional risk is the indirect adverse effects of chemotherapy. These include neutropenia, thrombocytopenia, cardiotoxicity, and increased risk of infection. Its effects on the fetus are difficult to assess and require further investigation. As conclusions, CC is rare in pregnant women. Gastrointestinal symptoms are common in them, but cancer warning signs (anemia, lower gastrointestinal bleeding, or change in bowel habits) can also occur during pregnancy without complications. All these symptoms deserve attention in situations of persistence or increase in severity despite treatment. Diagnostic possibility of cancer must be taken into account. The diagnosis of CC is based on colonoscopic findings during the second or third trimester. Imaging studies (ultrasound and MRI) are helpful to assess the severity of the condition. Serum carcinoembryonic antigen concentrations may be helpful in evaluating treatment success and early detection of recurrence. The main treatment is tumor resection, followed by end-to-end anastomosis. The adjuvant treatment is based on chemotherapy, with side effects demonstrated in animal studies. Radiation therapy should not be used in pregnant women with CC.
REFERENCES
1. Luna-Abanto J, Payet E. Importancia y estado actual de los registros de cáncer de base poblacional en Perú. Rev Med Herediana. 2019;30(2):131-3. Doi: 10.20453/rmh.v30i2.3558 [ Links ]
2. McClements J, Fitzpatrick D, Campbell WJ, Gavin A. Changes in management and outcome of patients with rectal cancer in Northern Ireland: 1996-2006. Colorectal Dis. 2014;16(2):58-65. doi: 10.1111/codi.12484 [ Links ]
3. Virgilio E, Costa G, Fransvea P, Balducci G. Colorectal cancer in pregnancy: one disease, two patients. ANZ J Surg. 2013;83(7-8):595. doi: 10.1111/ans.12208 [ Links ]
4. Dahling MT, Xing G, Cress R, Danielsen B, Smith LH. Pregnancy- associated colon and rectal cancer: perinatal and cancer outcomes. J Matern Fetal Neonatal Med. 2009;22(3):204-11. doi: 10.1080/14767050802559111 [ Links ]
5. ASGE Standard of Practice Committee, Shergill AK, Ben-Menachem T, Chandrasekhara V, Chathadi K, Decker GA, et al. Guidelines for endoscopy in pregnant and lactating women. Gastrointest Endosc. 2012;76(1):18-24. doi: 10.1016/j.gie.2012.02.029 [ Links ]
6. Kocián P, de Haan J, Cardonick EH, Uzan C, Lok CAR, Fruscio R, et al. Management and outcome of colorectal cancer during pregnancy: report of 41 cases. Acta Chir Belg. 2019;119(3):166-75. doi: 10.1080/00015458.2018.1493821 [ Links ]
7. Kamani L, Achakzai MS, Ismail FW, Kayani F. Safety of Endoscopy and Its Outcome in Pregnancy. Cureus. 2019;11(12):e6301. doi: 10.7759/cureus.6301 [ Links ]
8. Jain C. ACOG Committee Opinion No. 723: Guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol. 2019;133(1):186. doi: 10.1097/AOG.0000000000003049 [ Links ]
9. Zielinski R, Searing K, Deibel M. Gastrointestinal distress in pregnancy: prevalence, assessment, and treatment of 5 common minor discomforts. J Perinat Neonatal Nurs. 2015;29(1):23-31. doi: 10.1097/JPN.0000000000000078 [ Links ]
10. Austin LM, Frush DP. Compendium of national guidelines for imaging the pregnant patient. AJR Am J Roentgenol. 2011;197(4):W737-46. doi: 10.2214/AJR.10.6351 [ Links ]
11. Kal HB, Struikmans H. Radiotherapy during pregnancy: fact and fiction. Lancet Oncol. 2005;6(5):328-33. [ Links ]
12. Nguyen CP, Goodman LH. Fetal risk in diagnostic radiology. Semin Ultrasound CT MR. 2012;33(1):4-10. doi: 10.1053/j.sult.2011.09.003 [ Links ]
13. Pereg D, Koren G, Lishner M. Cancer in pregnancy: gaps, challenges and solutions. Cancer Treat Rev. 2008;34(4):302- 12. doi: 10.1016/j.ctrv.2008.01.002 [ Links ]
14. Toppenberg KS, Hill DA, Miller DP. Safety of radiographic imaging during pregnancy. Am Fam Physician. 1999;59(7):1813-8, 1820. [ Links ]
15. Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, et al. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol. 2013;22(1):14-21. doi: 10.1016/j.suronc.2012.10.003 [ Links ]
16. Healey AJ, Bansi D, Dhanjal MK, Blunt D, Dawson P, Buchanan GN. Colorectal stenting: a bridge to both caesarean section and elective resection in malignant large-bowel obstruction in pregnancy: a multidisciplinary first. Colorectal Dis. 2011;13(8):e248-9. doi: 10.1111/j.1463-1318.2010.02429.x [ Links ]
17. Alonso-Lázaro N, Bustamante-Balén M, Pous-Serrano S, Braithwaite-Flores A, Ponce-Romero M, Argüello-Viudez L, et al. Insertion of self-expanding metal stent for treatment of malignant obstruction in a pregnant woman. Rev Esp Enferm Dig. 2014;106(3):216-9. [ Links ]
18. Moore RG, Miller MC, Eklund EE, Lu KH, Bast RC Jr, Lambert- Messerlian G. Serum levels of the ovarian cancer biomarker HE4 are decreased in pregnancy and increase with age. Am J Obstet Gynecol. 2012;206(4):349.e1-7. doi: 10.1016/j.ajog.2011.12.028 [ Links ]
19. Salani R, Billingsley CC, Crafton SM. Cancer and pregnancy: an overview for obstetricians and gynecologists. Am J Obstet Gynecol. 2014;211(1):7-14. doi: 10.1016/j.ajog.2013.12.002 [ Links ]
20. Mechery J, Ikhena SE. Cancer of the descending colon during pregnancy. J Obstet Gynaecol. 2007;27(3):311-2. [ Links ]
21. Xu Y, Kong B, Shen K. Adenocarcinoma of the ascending colon in a 31-year-old pregnant woman: A case report. Medicine (Baltimore). 2018;97(51):e13707. doi: 10.1097/MD.0000000000013707 [ Links ]
22. Schwarzman P, Baumfeld Y, Bar-Niv Z, Baron J, Mastrolia SA, Sheiner E, Mazor M, Hershkovitz R, Weintraub AY. The effect of non-obstetric invasive procedures during pregnancy on perinatal outcomes. Arch Gynecol Obstet. 2015;292(3):603- 8. doi: 10.1007/s00404-015-3689-y [ Links ]
23. Højgaard HM, Rahr H. Rectal cancer in a pregnant woman, a case report. Ugeskr Laeger. 2012;174(26):1827-8. [ Links ]
24. Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy: a registry study of 5405 cases. Am J Obstet Gynecol. 1989;161(5):1178-85. [ Links ]
25. How P, Stelzner S, Branagan G, Bundy K, Chandrakumaran K, Heald RJ, et al. Comparative quality of life in patients following abdominoperineal excision and low anterior resection for low rectal cancer. Dis Colon Rectum. 2012;55(4):400-6. doi: 10.1097/DCR.0b013e3182444fd1 [ Links ]
26. Khodaverdi S, Kord Valeshabad A, Khodaverdi M. A case of colorectal cancer during pregnancy: A brief review of the literature. Case Rep Obstet Gynecol. 2013;2013:626393. doi: 10.1155/2013/626393 [ Links ]
27. Peccatori FA, Azim HA Jr, Orecchia R, Hoekstra HJ, Pavlidis N, et al: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi160-70. doi: 10.1093/annonc/mdt199 [ Links ]
28. Miller K, Zawislak A, Gannon C, Millar D, Loughrey MB. Maternal gastric adenocarcinoma with placental metastases: what is the fetal risk? Pediatr Dev Pathol. 2012;15(3):237-9. doi: 10.2350/11-08-1074-CR.1 [ Links ]
29. Bresalier RS, Ho SB, Schoeppner HL, Kim YS, Sleisenger MH, Brodt P, et al. Enhanced sialylation of mucin-associated carbohydrate structures in human colon cancer metastasis. Gastroenterology. 1996;110(5):1354-67. [ Links ]
30. Kojima S, Sakamoto T, Nagai Y, Honda M, Ogawa F. Metachronous rectal metastasis from primary transverse colon cancer: a case report. Surg Case Rep. 2018;4(1):90. doi: 10.1186/s40792-018-0498-0 [ Links ]
31. Wählby L. Carcinoembryonic antigen, CEA, in colorectal cancer. An insensitive marker which may be excluded from follow-ups. Lakartidningen. 1997;94(18):1716-8. [ Links ]
32. Luna-Pérez P, Alvarado I, Labastida S, Sosa J, Barrientos FJ, Herrera L. The mechanisms of the dissemination and the treatment of ovarian metastases in colonic adenocarcinoma. Rev Gastroenterol Mex. 1994;59(4):290-6. [ Links ]
33. Kim DD, Park IJ, Kim HC, Yu CS, Kim JC. Ovarian metastases from colorectal cancer: a clinicopathological analysis of 103 patients. Colorectal Dis. 2009;11(1):32-8. doi: 10.1111/j.1463-1318.2008.01543.x [ Links ]
34. Irons R, McIntosh E, Hageboutros A, Warshal D, McClane S. Bilateral ovarian micrometastatic adenocarcinoma upon prophylactic oophorectomy concurrent with low anterior resection for rectal cancer. World J Surg Oncol. 2017;15(1):40. doi: 10.1186/s12957-017-1115-6 [ Links ]
35. Omranipour R, Abasahl A. Ovarian metastases in colorectal cancer. Int J Gynecol Cancer. 2009;19(9):1524-8. doi: 10.1111/IGC.0b013e3181a84011 [ Links ]
36. Abdalla N, Bizon M, Piórkowski R, Stanirowski P, Cendrowski K, Sawicki W. Does chemotherapy for gynecological malignancies during pregnancy cause fetal growth restriction? Biomed Res Int. 2017;2017:7543421. doi: 10.1155/2017/7543421 [ Links ]
37. Boere I, Lok C, Vandenbroucke T, Amant F. Cancer in pregnancy: safety and efficacy of systemic therapies. Curr Opin Oncol. 2017;29(5):328-34. doi: 10.1097/CCO.0000000000000386 [ Links ]
38. Eastwood-Wilshere N, Turner J, Oliveira N, Morton A. Cancer in pregnancy. Asia Pac J Clin Oncol. 2019;15(6):296-308. doi: 10.1111/ajco.13235 [ Links ]
39. Selig BP, Furr JR, Huey RW, Moran C, Alluri VN, Medders GR, et al. Cancer chemotherapeutic agents as human teratogens. Birth Defects Res A Clin Mol Teratol. 2012;94(8):626- 50. doi: 10.1002/bdra.23063 [ Links ]
40. Leslie KK, Koil C, Rayburn WF. Chemotherapeutic drugs in pregnancy. Obstet Gynecol Clin North Am. 2005;32(4):627- 40. [ Links ]
41. Ngu SF, Ngan HY. Chemotherapy in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2016;33:86-101. doi: 10.1016/j.bpobgyn.2015.10.007 [ Links ]
42. Hankins GD, Clark SM, Munn MB. Cesarean section on request at 39 weeks: impact on shoulder dystocia, fetal trauma, neonatal encephalopathy, and intrauterine fetal demise. Semin Perinatol. 2006;30(5):276-87. [ Links ]
43. Cardonick E, Usmani A, Ghaffar S. Perinatal outcomes of a pregnancy complicated by cancer, including neonatal follow- up after in utero exposure to chemotherapy: results of an international registry. Am J Clin Oncol. 2010;33(3):221-8. doi: 10.1097/COC.0b013e3181a44ca9 [ Links ]
44. Gwyn K. Children exposed to chemotherapy in utero. J Natl Cancer Inst Monogr. 2005;(34):69-71. [ Links ]
45. Van Calsteren K, Berteloot P, Hanssens M, Vergote I, Amant F, Ganame J, et al. In utero exposure to chemotherapy: effect on cardiac and neurologic outcome. J Clin Oncol. 2006;24(12):e16-7. [ Links ]
46. Brent RL. Environmental causes of human congenital malformations: the pediatrician's role in dealing with these complex clinical problems caused by a multiplicity of environmental and genetic factors. Pediatrics. 2004;113(4 Suppl):957-68. [ Links ]
47. Jeppesen JB, Osterlind K. Successful twin pregnancy outcome after in utero exposure to FOLFOX for metastatic colon cancer: a case report and review of the literature. Clin Colorectal Cancer. 2011;10(4):348-52. doi: 10.1016/j.clcc.2011.06.003 [ Links ]
Received: February 21, 2020; Accepted: September 14, 2020











 texto em
texto em