Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.4 Lima oct-dic 2020
http://dx.doi.org/10.31403/rpgo.v66i2289
Case Report
Prenatal diagnosis of noncompaction of the ventricular myocardium: A case report
1 Professor of Obstetrics, University of Carabobo, Valencia, Venezuela; Perinatal Diagnosis Unit, Polyclinic Center Valencia, Venezuela.
"Spongy cardiomyopathy" or noncompaction heart disease is a rare congenital cardiomyopathy of unknown etiology which results from a failure in embryogenesis in the evolutionary process of normal myocardial trabeculation. The characteristic echocardiographic findings of this disease consist of multiple myocardial trabeculations and deep intertrabecular recesses that communicate with the left or right ventricular cavity or both. We present a case of this cardiomyopathy that affects the right ventricle, in a fetus of 31 weeks gestation, whose mother was an asymptomatic carrier of said pathology. Prenatal ultrasound images are presented, including evaluation using the strain (myocardial deformation) technique, emphasizing the importance of the patient’s medical history, possible etiologies and differential diagnosis with other entities.
Key words: Cardiomyopathy; non-compacted; Isolated noncompaction of the ventricular myocardium; Prenatal diagnosis; Ultrasound
Introduction
The 'spongy myocardium' is a rare congenital myocardiopathy of unknown etiology that results from arrest in normal endomyocardial embryogenesis. The characteristic echocardiographic findings of this disease consist of multiple myocardial trabeculations and deep intertrabecular recesses communicating with the left or right ventricular chamber or both1. This cardiomyopathy appears to divide the ventricular cavity, splitting its interior. It can present in prenatal, neonatal, infantile and adult forms, in which the spongy myocardium and systolic dysfunction are the common feature.
Prenatal diagnosis has been sporadically reported. Its frequency has been estimated at 0.12 per 100 000 or 1 in 850 000 children aged 0 to 10 years2. Histologically, it is characterized by poorly organized bundles of myocytes in the subepicardial and middle myocardial areas of both ventricles, and myocytes show thin fibers, often angulated, with a prominent clear center3. Studies in mouse embryos, using episcopic microscopy, have made it possible to evaluate the process of changes in endocardial trabeculation(4), although these results cannot be extrapolated to the human heart.
We present a case of this rare entity that affects the right ventricle, in an intrauterine patient with 31 weeks gestation, whose mother had this pathology and whose diagnosis oriented the investigation towards the fetus. Two-dimensional prenatal ultrasound and color Doppler images are shown, including evaluation using the myocardial deformation (strain) technique, emphasizing the importance of the patient's clinical history, differential diagnosis with other entities and its possible etiopathology mechanism.
Case Presentation
A 31-year-old patient, gesta III, para I, who referred, in previous gestation, stillbirth at 32 weeks. The patient's father had sudden death at age 40. The patient was a carrier of noncompaction heart disease with sporadic arrhythmias, asymptomatic at the time of the examination. The specialist's report oriented towards diagnostic investigation in the fetus. The current pregnancy was 31 weeks, fetal growth according to gestational age (AEG), with normal amniotic fluid index (ILA); normal fetal anatomy, female genitalia, with a slight pericardial effusion smaller than 3 mm. No screening was performed at 11-14 weeks. The antiphospholipid syndrome (APS) was ruled out with laboratory tests. She had a full-term delivery, live newborn Apgar 7 and 10, weight 3 100 g.
The diagnosis of asymptomatic noncompaction right ventricular cardiomyopathy was confirmed. He is currently in control. The images leading to diagnosis are shown. The rest of the fetal heart study, connections, arches, rhythm, and flowmetry were normal. The equipment used was Esaote MyLab Twice with a 2.5-3.5 MHz PA240 multifrequency transducer, and XStrain™ Doppler 2D software, with the ultrasonography equipment connected to an electrocardiographic signal generator, adjustable to the fetal heart rate detected by the Doppler flow wave of the mitral valve. Video recordings of three cardiac cycles were obtained in a four-chamber plane, and those in which the zero-voltage curve corresponded to the S wave of the EKG QRS were processed. The regional patterns of myocardial deformation were completely different, being evident the altered deformation (strain) curves of the presented case, with respect to the observed unaffected case (control). Figures 1 and 2.
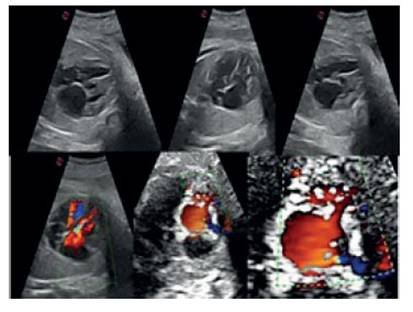
Figure 1 Sequence of tetrachameral section images of the Fetal heart, showing the asymmetry of the chambers at the expense of the right ventricle, in which numerous trabeculations and blood flow (color doppler) are obServed in the cardiac chamber, penetrating the intertrabecular recesses.
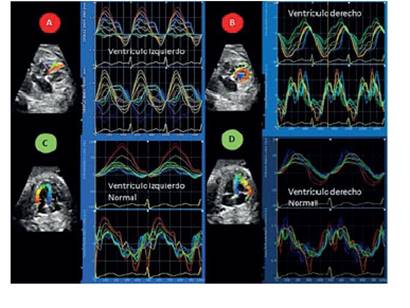
Figure 2 Strain curves and their respective strain rate (strain and strain rate) of both ventricles of the case with noncompaction cardiomyopathy (red circles a and b), with obvious differences when compared with a normal control case (green circleS c and d) of the Same gestational age (31 weeks). the regional patterns of myocardial deformation were completely different in the right ventricle of the case compared to that observed in the unaffected heart (control).
Discussion
The bibliography consulted1-19 summarizes the possible causes of this entity, among which are proteins with genetic mutations, probably responsible for the appearance of cases with noncompaction of the left ventricle (NCLV), genes responsible for familial incidence, X-linked family disorders, mutated genes, and genotype-phenotype correlations of various syndromes such as Becker muscular dystrophy, Emery-Dreifuss muscular dystrophy or Barth syndrome. The latter is characterized by neutropenia, growth retardation, high urinary organic acids, low carnitine levels, associations with other entities, such as mitochondrial disorders (DNAmt, DNA mutations). Furthermore, the CSX gene has been implicated in the development of some cases of noncompaction cardiomyopathy. The genetic background suggests that the uniform morphology of left ventricular hypertrophy could not only be attributable to embryonic non-compaction, but could also be the result of induction of hypertrabeculation, as a compensatory reaction of an impaired myocardium. Differential diagnosis includes, but is not limited to, left ventricular hypertrophy, Pompe disease, dilated cardiomyopathy, apical hypertrophic cardiomyopathy, endocardial fibroelastosis, left ventricular thrombus, and arrhythmogenic diseases.
This cardiomyopathy was first described in 1984 by Engberding and Bender14. Previously, this type of abnormality had been recognized only in cases of congenital heart defects with an intact ventricular septum and the semilunar valve atresia. Engberding and Bender called the disorder “persistence of isolated myocardial sinusoids”. It can occur as an isolated entity or associated with other heart pathologies11, and can often affect both ventricles.
Diagnosis is done by echocardiography. Jenni et al.(13) defined criteria for echocardiographic diagnosis of left ventricle noncompaction (LVNC), and these are: 1) coexisting cardiac abnormalities caused by exposure to high pressure of the ventricle during intrauterine development are absent, and in which various forms of semilunar valve obstruction are absent; 2) the left ventricular wall is thickened and consists of a 2-layer structure: a compacted epicardial band of uniform tissue and a much thicker, non-compacted endocardial layer of prominent trabeculations and deep intertrabecular recesses, with a relationship during maximum systole between layers not compacted to compacted greater than 2, these characteristics are found predominantly in the apical and middle ventricular segments of the left ventricle; and 3) color Doppler shows blood flow passing directly from the ventricular cavity into the spaces between prominent trabeculations, and is visualized throughout the heart cycle. A noncompacted ventricular wall (NC) over compacted wall (PC) ratio greater than 3 and involvement of three or more segments in the ventricular chamber are signs of poor prognosis, associated with greater clinical deterioration (functional class III/IV) and ventricular arrhythmias.
The issue has been open to controversy. Thus, Anderson(5) believes that the evidence is growing that noncompaction left ventricular cardiomyopathy, as currently defined, does not represent a preexisting trabecular myocardial compaction failure found during embryonic development to form the compact component of the ventricular walls. Furthermore, this author5 states that there is no evidence that we are aware of favoring the notion that the entity is a return to a phenotype seen in cold-blooded animals, adding that it is known that, when observed in adults, the presence of excessive ventricular trabeculations does not augur a poor prognosis when the ejection fraction is normal. The review carried out by this author suggests that the presence of an excessively trabeculated left ventricular wall is not in itself a clinical entity and, therefore, maintains that the term “noncompaction cardiomyopathy” is misleading, because there is no compaction failure or a cardiomyopathic process in most individuals who meet widely used diagnostic criteria.
An interesting contribution, pending to be corroborated in humans, is the findings of episcopic microscopy, which has allowed the trabecular maturation process to be studied in detail4, which is summarized by Sun et al. as follows: in the fourth week of normal embryonic development, the myocardium is composed of a spongy reticular layer before the formation of the coronary artery circulation. Between 5 and 8 weeks, the ventricular myocardium gradually becomes compact. The compaction process begins from the epicardium to the endocardium, from the base to the apex. As a result, the trabeculae are constantly absorbed and compacted, with the subsequent progressive expansion of the ventricular cavity. At 12 weeks of embryonic development, the compaction process is complete. The myocardium near the epicardium is fully formed, while the myocardium near the endocardium is not as fully formed as the one near the epicardium. Consequently, some pectinate muscles and trabeculae remain. The smooth part of the interventricular septum is so named because of the absorption of more complete trabeculae from the upper segment of the interventricular septum, while the lower segment of the septum, due to incomplete absorption, is called the trabecularized portion of the lower segment of the ventricular septum.
Important information is gathered in the reviews4,6,7,14, among which it stands out that left ventricular noncompaction is diagnosed in 0.05%-0.26% of adult patients referred for echocardiographic examinations, with a male predominance. However, some studies report a prevalence of 0.01%-1.3% in the general population. In patients affected by noncompaction of the left ventricle (NCLV), this entity is the cause of heart failure in 3-4/100 affected. The rate of family involvement seems to vary between 18 and 33%. Left ventricular noncompaction is a unique inherited cardiomyopathy, characterized by an increased risk of adverse cardiovascular events, such as heart failure, arrhythmia or sudden cardiac death.
Although most of the published cases correspond to left ventricular noncompaction, Tian et al.(6 examined nine cases of this pathology in fetuses, using prenatal echocardiography, and found that the trabeculated myocardium and noncompaction/compaction ratio (N/C ratio) ≥2.0 was the echocardiographic characteristic that allowed diagnosis. In the nine fetuses with prenatal diagnosis, six were diagnosed with left ventricular noncompaction, two with noncompaction of both ventricles (biventricular NC) and one with right ventricular noncompaction. Muscle biopsies were performed in three of the aborted fetuses and abnormal mitochondria, sarcomeres and myocardial fibers were observed. These authors6conclude that noncompaction can be identified in the fetus, and that the most frequent one involves the left ventricle, but can also affect the right ventricle, or even both ventricles. The myocardial ultrastructure of fetal ventricular noncompaction has certain unique characteristics with respect to the maturation of mitochondria, sarcomeres and myocardial fibers.
Karatza et al.9) state that patients, depending on the extent of the lesion, may be asymptomatic or present symptoms of heart failure, systemic embolization or various forms of arrhythmias. Some patients show a characteristic dysmorphic facial appearance represented by a prominent forehead, lowered ears, high arched palate and micrognathia. In these authors, the increased nuchal fold led them to suspect a chromosomal abnormality. However, amniocentesis revealed, in the two cases studied, a normal female karyotype. The female sex also excluded the possibility of Barth syndrome.
For Engberding et al.(14, diagnosis is based on the following echocardiographic criteria: the presence of at least 4 prominent trabeculations and deep intertrabecular recesses, blood flow from the ventricular cavity to the intertrabecular recesses, and a bilaminar structure typical of the affected portion of the left ventricular myocardium. Also, it can be diagnosed with magnetic resonance imaging of the heart.
The clinical manifestations of the condition and its severity are variable and include heart failure, thromboembolic events and arrhythmias. Treatment is based on symptoms. Symptomatic carrier patients have a poor prognosis. These authors14 attribute to Chin et al.(15 the suggestion that the entity be renamed as “isolated left ventricular myocardium noncompaction”, based on a better understanding of developmental physiology, emphasizing that this designation implies an alteration of the compaction process that occurs normally, as part of embryonic morphogenesis of the myocardium.
Clinical manifestations in the fetus and newborn have been published, such as increased nuchal translucency9, ascites and congestive heart failure3, arrhythmias, hydrops, sudden death, facial dysmorphism (frontal dome, lowered ears, strabismus and micrognathia)10,12,13). Another important clinical finding is the coexistence of some types of neuromuscular disorder and association with Barth syndrome19.
As reported by Xiao-Jin Ma et al.(3, echocardiography can be used in qualitative and quantitative diagnosis of noncompaction cardiomyopathy and in the evaluation of cardiac function. The apex and the midsegment of the left ventricular lateral wall are often involved, accompanied by a decrease in the left ventricular ejection fraction. Heart failure was found in 83% (44/53) of children with this pathology, and the left ventricular ejection fraction for these children was 43 ± 9%.
In addition to the strain technique, the strain rate has been used in adults to assess myocardial deformation in the different segments of the affected cavity(16.17), finding a reduction in strain rates especially in mid-ventricular and apical cuts affected. The deterioration of all the deformation parameters and the strain rate correlates well with the extension of the non-compacted myocardium. Magnetic resonance imaging (MRI) has shown utility in the diagnosis18.
In the reported case, the regional patterns of myocardial deformation were completely different, being evident the altered strain curves of the case with respect to that observed in the unaffected heart, especially those of the right ventricle.
Conclusions
Noncompaction cardiomyopathy is a rare entity that can lead to serious compromise of the affected individual’s health, and it can be suspected in the prenatal stage in the presence of marked cardiomegaly or of the left, right or both, dilated and hypertrophic ventricle, the presence of numerous trabeculations and deep intertrabecular recesses within the cardiac ventricular myocardium. These show direct blood flow from the ventricular cavity to the deep intertrabecular spaces, which is evidenced by color Doppler. In the present case, it was an antenatal case of noncompaction of the right ventricle, whose mother was a carrier of said pathology, and which was used for the exhaustive study of her baby’s heart by means of 2D echocardiography, color Doppler and curves of myocardial deformation (strain), the latter which, according to the bibliography, has not been applied in the neonatal stage in the diagnosis of this entity.
We consider that the prenatal diagnosis, the evaluation of the cardiac performance of the fetal heart, the specialized evaluation at the time of birth, will allow the adequate management of each case, as well as establishing an adequate prognosis for each patient. This is crucial to implementing the most appropriate treatment, and should be based on the results of a variety of clinical trials.
Gratitude
To Dr. Gonzalo Moscoso for providing important support information.
REFERENCES
1. Captur G, Wilson R, Bennett MF, Luxán G, Nasis A, de la Pompa JL, et al. Morphogenesis of myocardial trabeculae in the mouse embryo. J Anat. 2016;229(2):314-25. doi:10.1111/ joa.12465 [ Links ]
2. Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Características ecocardiográficas y patoanatómicas de la no compactación ventricular aislada: un paso hacia las clasificaciones como una miocardiopatía distinta. Corazón. 2001;86:666-71. [ Links ]
3. Ma X-J, Huang GY, Zhang J, Gao Y, Liang XC, Chen WD. Zhongguo Dang Dai Er Ke Za Zhi. [Diagnosis of noncompaction of the ventricular myocardium by echocardiography]. 2015;17(10):1074-8. [ Links ]
4. Kubik M, Dabrowska-Kugacka A, Lewicka E, Danilowicz-Szymanowicz L, Raczak G. Predictors of poor outcome in patients with left ventricular noncompaction: Review of the literature. Adv Clin Exp Med. 2018;27(3):415-22. doi:10.17219/acem/67457 [ Links ]
5. Anderson RH, Jensen B, Mohun TJ, Petersen SE, Aung N, Zemrak F, et al. Key questions relating to left ventricular noncompaction cardiomyopathy: is the emperor still wearing any clothes? Can J Cardiol. 2017;33(6):747-57. doi:10.1016/j.cjca.2017.01.017 [ Links ]
6. Tian L, Zhou Q, Zhou J, Zeng S, Cao D, Zhang M. Ventricular non-compaction cardiomyopathy: prenatal diagnosis and pathology. Prenat Diagn. 2015;35(3):221-7. doi:10.1002/pd.4523 [ Links ]
7. Nappi L, Vasciaveo L, Sorrentino F, Scutiero G, Iannone P, Greco P. Fetal noncompaction cardiomyopathy and histologic diagnosis of spongy myocardium: Case report and review of the literature. Rev Bras Ginecol Obstet. 2018;40(11):722-5. doi:10.1055/s-0038-1673677 [ Links ]
8. Zhou J, Tian L, Zhou Q, Zeng S, Zhou J, Zhang R, Tong H. [Echocardiographic diagnosis for ventricular non-compaction cardiomyopathy in foetus and the pathologically comparative study]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;40(7):754-9. doi:10.11817/j.issn.16727347.2015.07.009 [ Links ]
9. Sun L, Zhao E, Wei Y, Kang C, Liu B. Thickness and ratio of noncompacted and compacted layers of the left ventricular myocardium evaluated in 56 normal fetuses by two-dimensional echocardiography. Biomed Res Int. 2019;2019:3726846. doi:10.1155/2019/3726846 [ Links ]
10. Karatza AA, Holder SE, Gardiner HM. Isolated non-compaction of the ventricular myocardium: prenatal diagnosis and natural history. Ultrasound Obstet Gynecol. 2003;21(1):7580.doi:10.1002/uog.10 [ Links ]
11. Zhang J, Wang Y, Feng W, Wu Y. Prenatal ultrasound diagnosis of fetal isolated right ventricular noncompaction with pulmonary artery sling: A rare case report. Echocardiography. 2019;36(11):2118-21. doi:10.1111/echo.14528 [ Links ]
12. Tomar M, Radhakrishnan S. Biventricular non compaction: A rare cause of fetal distress and tricuspid regurgitation. Images Paediatr Cardiol. 2009;11(4):1-5. [ Links ]
13. Muraoka J, Kodama Y, Sameshima H, Michikata K, Matsuzawa S, Masanao O, Kaneko M, akaki M, Sato Y. Fetal left ventricular non-compaction cardiomyopathy with ascites: A case report. J Obstet Gynaecol Res. 2017;43(9):1481-4. doi:10.1111/jog.13381 [ Links ]
14. Engberding R, Stöllberger C, Ong P, Yelbuz TM, Gerecke BJ, Breithardt G. Isolated non-compaction cardiomyopathy. Dtsch Arztebl Int. 2010;107(12):206-13. doi:10.3238/arztebl.2010.0206 [ Links ]
15. Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R: Isolated non compaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82:507-13. [ Links ]
16. Gastl M, Gotschy A, Polacin M, Vishnevskiy V, Meyer D, Sokolska J, Tanner FC, et al. Determinants of myocardial function characterized by CMR-derived strain parameters in left ventricular non-compaction cardiomyopathy. Sci Rep. 2019;9(1):15882. doi:10.1038/s41598-019-52161-1 [ Links ]
17. Niemann M, Liu D, Hu K, Cikes M, Beer M, Herrmann S, et al. Echocardiographic quantification of regional deformation helps to distinguish isolated left ventricular non-compaction from dilated cardiomyopathy. Eur J Heart Fail. 2012;14(2):155-61. doi:10.1093/eurjhf/hfr164 [ Links ]
18. Choi Y, Kim SM, Lee SC, Chang SA, Jang SY, Choe YH. Quantification of left ventricular trabeculae using cardiovascular magnetic resonance for the diagnosis of left ventricular non-compaction: evaluation of trabecular volume and refined semi-quantitative criteria. J Cardiovasc Magn Reson. 2016;18(1):24. doi:10.1186/s12968-016-0245-2 [ Links ]
19. Clarke SL, Bowron A, Gonzalez IL, Groves SJ, Newbury-Ecob R, Clayton N, et al. Barth syndrome. Orphanet J Rare Dis. 2013;8:23. doi:10.1186/1750-1172-8-23 [ Links ]
Received: July 17, 2020; Accepted: September 01, 2020











 texto en
texto en 



