Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.66 no.4 Lima oct-dic 2020
http://dx.doi.org/10.31403/rpgo.v66i2291
Case Report
Caudal agenesis with closed spinal dysraphism, a case report
1 Obstetrician gynecologist, Daniel Alcides Carrión National Hospital, Callao, Perú; Fetal Medical Centre: CENMEF; Maternal Fetal Medicine Group Fetalis.
2 Resident in Gynecology and Obstetrics, Daniel Alcides Carrión National Hospital, Callao, Perú.
Caudal agenesis is a rare pathology, its diagnosis and fetal evaluation are complex. The presence of closed spinal dysraphism at the level of the sacrum should compel us to evaluate the anatomy of the sacrum. The evaluation of the medullary cone is very useful to evaluate and exclude closed spinal dysraphism and caudal agenesis. We report a rare case of caudal agenesis.
Key words: Sacral agenesis; Spinal dysraphism; Spinal cord; Congenital anomalies
Introduction
Caudal agenesis is a rare disorder that involves multiple caudal structures. The approximate incidence is 0.01 to 0.05 per 1, 000 liveborn. It has been related to pre-pregnancy diabetes as a risk factor (1 per 350 liveborn)1). It has many denominations, such as sacral agenesis or caudal regression, but the pathophysiology goes beyond sacral bone affection or isolated regression process. Therefore, the correct denomination is still controversial2).
Although, the most severe defects can be diagnosed in the earliest years of life or prenatally, most of them are detected during childhood and adolescence. This is due to the great variety of phenotypic presentations. One of the most frequent associations is with closed spinal dysraphism. A prenatal case of caudal agenesis associated with closed spinal dysraphism is presented and the literature is reviewed.
Case Report
A 28-week primigravida was transferred to the hospital due to suspicion of spina bifida. She denied history of pre-pregnancy diabetes. Ultrasound showed a fetus in podalic presentation with a huge tumor at the lumbosacral region with cystic pattern and covered with skin and trabeculations that corresponded with neural tissue. It was possible to visualize the spinal cord down to the level of the lesion in the sacral region; vertebrae could not be seen optimally (Figures 1 and 2). The diagnosis of closed spinal dysraphism was made, with suspicion of sacral agenesis. The evaluation of the fetal skull and brain did not find alterations; normal posterior cerebral fossa, left renal agenesis, and rest of anatomical evaluation within normal. Fetal biometry was adequate for gestational age.
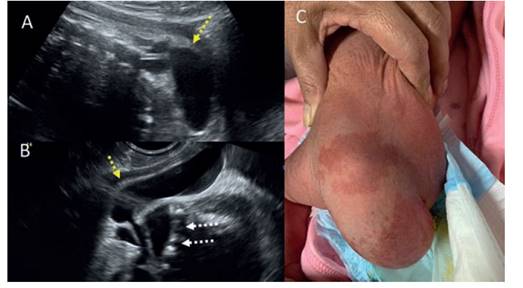
Figure 1 A: Sagittal section at the level of the lumbosacral column showing anechoic tumor covered with skin (yellow arrow). b: coronal section at the level of the sacral column; white arrows show sacrAl vertebral bodies and anechoic tumor with neural tissue inside covered with skin (yellow arrow). c: photograph of the lumbosacral region of the newborn, with a predominAntly left tumor with alterations in skin coloration.
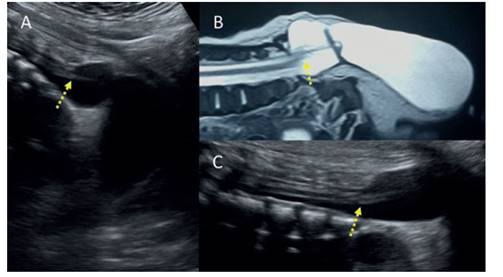
Figure 2 A: Sagittal section of the lumbosacral spine showing the level of the medullary cone thinned, reaching the closed dysraphism. b: resonance of the sacral spine revealing how the medullary cone is fixed to the neural defect, with marked thinning. this can also be shown in the ultrasound image in c. the yellow Arrow marks the medullary cone anchored at the level of the sacrum and with abnormal morphology.
Delivery was by cesarean section at 38 weeks of gestation of a 2 865 g female newborn, Apgar score 9-9. The newborn presented a soft tissue tumor of 10 x 8 cm approximately (Figure 1C) covered with skin at the sacrum level and the left leg was equinovarus with muscle hypotrophy and monoparesis. There were no anorectal malformations. Hip radiography showed alteration of both femoral heads and acetabula, with predominance in the left hip, and absence of the left hemisacrum from S2 (Figure 3A). Tomography of the brain was normal. Abdomen ultrasound showed left renal agenesis. Spine resonance showed a skin covered meningocele with an extensive defect in the posterior arches and hemisacrum from S2, S3, S4 and S5, with protrusion of the dural sac, which extended into the perineal region. The low implantation spinal cord was determined down to the S2 level. Hipotrophy was detected in the left iliopsoas muscles, thigh and gluteus (Figure 4). Review of the prenatal images showed agenesis of the left hemisacrum (Figure 3B), which was correlated with the hips radiographs.
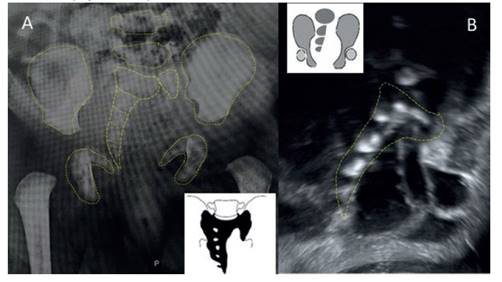
Figure 3 A: newborn hip radiograph shows the right hemisacrum with persistence of abnormal-looking s1, but the left side of the vertebra is still visible. it would correspond to a type iv b (unilateral subtotal hemisacrum) of the pang classification (box drawing). b: coronal fetal ultrasound section showing the right hemisacrum. unilateral sacral agenesis, according to renshaw’a aimplified classification (inset drawing).
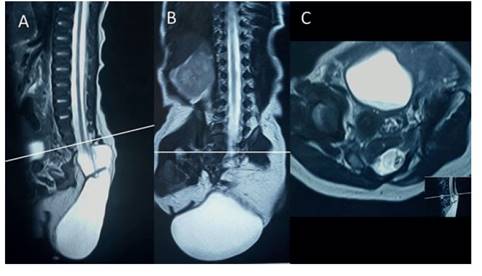
Figure 4 A: resonance sagital section showing Absence of the sacrum. the meningocele covered by skin is observed, with an abnormal medullary cone fixed to the lesion. b: coronal section of the column partially showing the sacral vertebrAl bodies on the right side (yellow arrow). c: axial section at the level of s1 shows the vertebral body with an abnormal appearance, with a recognizable right side (yellow arrow), but the left hemivertebra is hypoplastic. white line at S1 level.
She underwent meningocele plasty, finding two cystic cavities, one extensive with citrine fluid and the other small with clear fluid, in continuation both with the intraspinal and subarachnoid space. The nerve roots were contained in the intraspinal space, but protruded by default. The baby had an infection at the surgical site and was discharged 42 days after surgery.
Discussion
Caudal agenesis is a complex disorder of spinal development, which not only involves alterations of the sacrum and coccyx, but also with neural, gastrointestinal and urological components. It has its embryological origin in the alteration of the development of the caudal eminence, the caudal notochord and secondary neurulation, which explains the great variety of phenotypes of the disease1-3. It can present as partial or total absence of the vertebral bodies of the coccyx, sacrum, lumbar or thoracic spine, with a variable and inconstant degree of spinal, nephrourological, anorectal or cardiac malformations.
Sometimes it is part of syndromes such as VATER (vertebral anomalies, anal atresia, tracheo-esophageal fistula, renal/radial anomalies), OEIS (omphalocele, cloacal exstrophy, imperforate anus, spinal defects), and Currarino (sacral agenesis, presacral mass and anorectal anomaly triad)(1-3.
This disease can be classified in two types, considering it as a complex spinal dysraphism (when the medullary shape is assessed): type I abrupt termination of the medullary cone with great involvement of the lower vertebral bodies and type II - as anchored, extended and thin spinal cord, usually associated with an intraneural lipoma, terminal myelocystocele or lipomyelomeningocele4. Other frequently used classifications are Renshaw's (1978)5) and Pang's (1993) 6, both based on the radiological appearance of the sacrum (total, partial hemisacral agenesis or variants) and its relationship with the iliac bones and lumbar spine. Figure 3 shows the comparison of both classifications applied to the case.
Prenatal ultrasound evaluation, in case of a suspicion of caudal agenesis, should be based on: fetus of a diabetic mother, sacral spinal dysraphism or fetal syndromes of known association with the disease. If possible, we must determine the type of sacrum agenesis by volumetric reconstruction of the bone. We recommend in prenatal diagnosis to use Mottet’s proposal7, which is a Renshaw simplification, in type I: partial or total unilateral agenesis, type II: asymmetric partial bilateral agenesis, and type III: total agenesis. Perform a systematic search for closed spinal dysraphisms by visualizing the fetal medullary cone (position: anchored medullary cone; and shape: abrupt or truncated medullary cone) (Figures 2 and 4). Then, make a systematic review of associated malformations: gastrointestinal, nephrourological and spinal (hemivertebral or deformities), and particularly the anorectal malformations1.
Multiple reports of isolated cases of prenatal diagnosis have been published, but the most extensive fetal series are related to closed spinal dysraphism. Morel(8) reports 19 cases collected in 9 years in a single referral center, classifying sacral agenesis as partial or total: 12 cases were partial and 2 total agenesis. He also considered 4 cases of abnormalities of segmentation (sacral hemivertebra) and one of angulation disturbance. Fourteen cases presented closed spinal dysraphism, 8 cases with anchored medullary cone, 5 with truncated medullary cone, and 1 terminal filum lipoma. In associated defects, 8 cases presented anorectal malformations and 5 nephrourological.
In a retrospective series of 10 years of prenatal diagnosis, Mottet7 presents 10 cases and relates them to alterations in the visualization of the fetal medullary cone. He classifies the agenesis according to a Renshaw simplification applied to prenatal diagnosis (Figure 3B), finding 40% with anchored medullary cone and 20% truncated medullary cone. 90% of the cases presented closed spinal dysraphism. Most of the cases with a medullary cone in normal position (medullary level) were associated with spinal lipoma. The defects of other systems were, with high frequency, anorectal and nephrourological malformations, two cases were classified as Currarino syndrome and one as VACTER.
The association between spinal dysraphism and caudal agenesis is also described in pediatric series. Balioglu9 reported 20 cases of closed spinal dysraphism in 38 cases of caudal agenesis; 45% had an anchored medullary cone and 20% had a terminal filum lipoma/lipoma. Likewise, Enami-Naeni, in a cohort of 50 patients, found 44 with some type of spinal dysraphism, anchored spinal cone in 29 cases and 12 with lipomyelomeningocele10. Jeelani, in the 17-year retrospective analysis of pediatric patients with caudal agenesis with closed spinal dysraphism, reports 22 cases, 13 diagnosed in the first year of life, mainly in relation to VACTER or OEIS. The most frequent closed dysraphism was the anchored medullary cone (14 cases), mainly presenting in adolescents. On the other hand, lipomatous malformations with anchored medullary cone (8 cases) were diagnosed in children under one year.
Finally, in the event of any lumbo-sacral spinal dysraphism (especially closed ones), we must evaluate the sacrum, in search of caudal agenesis. Limitations in the ultrasound visualization of the sacrum prenatally make it advisable to use the simplified Renshaw classification. It is very difficult to establish neurological or locomotion prognosis in the prenatal evaluation.
REFERENCES
1. Mottet N, Chaussy Y, Auber F, Guimiot F, Arbez-Gindre F, Riethmuller D, et al. How to explore fetal sacral agenesis without open dysraphism key prenatal imaging and clinical implications. J Ultrasound Med. 2018;37(7):1807-20. DOI:10.1002/jum.14522 [ Links ]
2. Lee JY, Pang D, Wang KC. Caudal agenesis and associated spinal cord malformations. In: Di Rocco, Concezio, Pang, Dachling, Rutka, James T (Eds.). Textbook of Pediatric Neurosurgery. Springer International Publishing 2017. DOI 10.1007/978-3-319-31512-6_119-1 [ Links ]
3. Jeelani Y, Mosich GM, McComb JG. Closed neural tube defects in children with caudal regression. Childs Nerv Syst 2013;29:1451-7 DOI 10.1007/s00381-013-2119-3 [ Links ]
4. Tortori-Donati P1, Rossi A, Cama A. Spinal dysraphism: a review of neuroradiological features with embryological correlations and proposal for a new classification. Neuroradiology. 2000;42(7):471-91. DOI: 10.1007/s002340000325 [ Links ]
5. Renshaw TS. Sacral agenesis. J Bone Joint Surg Am. 1978;60(3):373-83 [ Links ]
6. Pang D, Hoffman HJ. Sacral agenesis with progressive neurological deficit. Neurosurgery. 1980;7:118-26. [ Links ]
7. Mottet N, Martinovic J, Baeza C, Guimiot F, Bault JP, Aubry MC, et al. Think of the conus medullaris at the time of diagnosis of fetal sacral agenesis. Fetal Diagn Ther. 2017;42:137- 43. DOI: 10.1159/000451080 [ Links ]
8. Morel B, Friszer S, Jouannic JM, Ducou Le Pointe H, Blondiaux E, Garel C. Prenatal sacral anomalies leading to the detection of associated spinal cord malformations. Fetal Diagn Ther. 2017;42:294-301. DOI: 10.1159/000457795 [ Links ]
9. Balioglu MB, Akman YE, Ucpunar H, Albayrak A, Kargin D, Atici Y, et al. Sacral agenesis: evaluation of accompanying pathologies in 38 cases, with analysis of long-term outcomes. Childs Nerv Syst. 2016;32(9):1693-702. doi: 10.1007/s00381-016-3022-5 [ Links ]
10. Emami-Naeini P, Rahbar Z, Nejat F, Kajbafzadeh A, El Khashab M. Neurological presentations, imaging, and associated anomalies in 50 patients with sacral agenesis. Neurosurgery. 2010;67(4):894-900. DOI: 10.1227/NEU.0b013e3181eb500d [ Links ]
Received: June 23, 2020; Accepted: August 12, 2020











 texto en
texto en 


