Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.67 no.1 Lima ene./mar 2021
http://dx.doi.org/10.31403/rpgo.v67i2301
Original Articles
Clinicopathological characteristics, treatment and prognosis of phyllodes tumor in reference centers in Medellín, Colombia
1 Mastologist Physician, Professor UniRemington, Medellin, Colombia; Cancer Research Group
Objective
: To describe the sociodemographic data, clinical and paraclinical manifestations, treatment and recurrence, among a series of patients diagnosed with phyllodes tumor.
Methods
: Descriptive, retrospective study of an institutional database, between January 1, 2016 and December 31, 2019. Demographic, clinical and paraclinical data were collected from medical records, which were analyzed using descriptive statistics.
Results
: The prevalence of phyllodes tumor in the sample was 1.3%. All cases were women, mean age 42 years, with breast mass as the most frequent reason for consultation (n = 42, 97.6%), mean tumor size 8.6 cm (range 2.1-20 cm). The sharp biopsy made the diagnosis of phyllodes tumor in 8 patients (18.6%) and fibroadenoma in 30.2% (n = 12). According to the histological classification, the most frequent were the benign and borderline type with 34.9% (n = 15) each, and malignant in 30.2% (n = 13). The type of surgery most performed was quadrantectomy in 55.8% (n = 24). The mean follow-up was 60.3 months and relapse occurred in 9.3% (N = 4) in about 23.2 months.
Conclusions
: In the sample studied, the phyllodes tumor affected women in the fourth decade of life, it appeared as a large, unilateral mass, without predominance of laterality, of rapid growth. The optimal treatment was surgery with wide negative margins, and radiotherapy in selected cases. The risk of recurrence is important.
Key words: Breast; Breast neoplasms; Phyllodes tumor
Introducción
Phyllodes tumor (PT) is a low prevalence breast cancer with an epithelial and stromal component, with a potential risk of recurrence1. It was first described with the term phyllodes cystosarcoma, by Johannes Müller, in 18382.
PT represents 0.3% to 0.9% of all breast tumors3, and 2% to 3% of fibroepithelial neoplasms of the breast4. In the general population, the annual incidence is 2.1 per million inhabitants, with a risk 3 to 4 times higher in Asian and Latina women5.
There may be a concomitance with fibroadenoma in 20%, or a previous history of fibroadenosis in 12.5%. No predisposing factors for phyllodes tumor have been found, with the exception of Li-Fraumeni syndrome6.
The mean age of presentation is 40 years, with a range between 14 and 82 years7. Clinically, it manifests as a single, unilateral breast mass, usually greater than 5 cm, painless, with a firm consistency, well defined, mobile and without compromising the skin or deep planes; bilateral lesions represent less than 2.5% of cases. The axillary nodes can be found reactive in 17% and with metastatic involvement in 1%8. Systemic spread is rare and mainly affects the lungs, liver, and brain8.
Macroscopically, the PT is rounded or lobed, firm, with well-circumscribed edges; when cut, it is greyish white8. Microscopically, a proliferative stroma lined by epithelium and benign glandular elements is observed, in a “leaf-like” shape, which gives it its name in Greek (phyllos = leaf) 9. Figure 1.
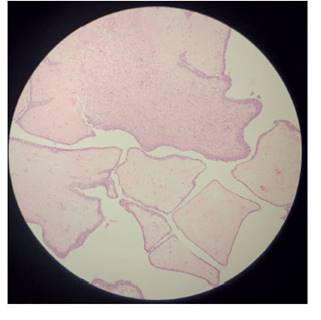
Figure 1 photomicrograph of the phyllodes tumor. it shows benign glandular elements, shaped “like a leaf”.
The most important differential diagnosis is fibroadenoma, the appearance and amount of stromal component being the factors to take into account10. Core needle biopsy may be incorrect or inconclusive in differentiating a benign phyllodes tumor from a fibroadenoma11.
According to histological parameters, such as the stromal characteristics of atypia, mitosis, hypercellularity, overgrowth and margins, they are classified as benign, borderline and malignant. Approximately 52% are benign, 13% intermediate malignancy, and 35% malignant12,13.
Treatment is surgical. Radicality depends on the breast-tumor relationship. It requires wide local excision with margins of at least 10 mm, which reduces the prevalence of recurrence14,15. Axillary lymph node dissection is not recommended14. In cases of malignant PT, radiotherapy has been used as adjuvant therapy16,17. No benefit has been shown with chemotherapy18.
The objective of this study was to make a retrospective review of clinical aspects, diagnosis, treatment and prognosis of patients who attended the mastology consultation with a diagnosis of phyllodes tumor. It is one of the largest case series published in Latin America.
Methods
This is a retrospective descriptive study of users seen in the mastology consultation at the Medellín Clinic, the Antioquia Oncology Center and the Profamilia Clinic in the city of Medellín, for the period January 1, 2016 and December 31, 2019.
Out of a total of 2 863 users seen, a search was made for medical records with the ICD code 10 D486 Tumor of uncertain or unknown behavior of the breast. 48 records were identified, creating an independent database for this cohort of patients. Five cases that did not meet the diagnostic criteria were excluded, and a subgroup analysis of the 43 cases diagnosed with phyllodes tumor was performed, obtaining demographic data and clinical and pathological characteristics. Data on age, reason for consultation, findings on physical examination, affected side, findings on ultrasound and mammography, types of treatment, such as quadrantectomy, mastectomy, follow-up and recurrence were included. The exclusive diagnostic criterion was the histopathological study by sharp needle biopsy or the study of the surgical specimen of phyllodes tumor. Patients with loss of relevant information from clinical records were excluded.
Descriptive analysis of sociodemographic and clinical variables was performed. Absolute frequencies and percentages, mean and standard deviation were calculated, according to the nature and distribution of the variables. The normal distribution was validated using the Shapiro Wilk goodness of fit test. A bivariate analysis was performed with the chi2 test to establish the association between recurrence and treatment modalities and risk factors. For the hypothesis tests, a 95% confidence interval and a 5% significance level were set. A recurrence curve was performed according to histological type, using Kaplan Meier curves, which were compared by the log-rank test. SPSS statistics version 23 software was used.
Regarding ethical aspects, this is considered a risk-free study, according to the classification set forth in Article 11 of Resolution No. 008430 of 1993 (issued by the Ministry of Health of Colombia), and is in line with international regulations, Declaration of Helsinki, and the ethical guidelines for biomedical research prepared by the Council for International Organizations of Medical Sciences -CIOMS.
Results
A total of 2 863 users were seen in the period studied. 48 records were identified with the diagnosis of ICD code 10 D486 Tumor of uncertain or unknown behavior of the breast, and 34 met the inclusion criteria for phyllodes tumor. For this cohort, the prevalence was 1.3% (Figure 1).
All cases (n = 43) occurred in women with a mean age of 42 years (SD 13.1. Range 17 to 85). The majority resided in Medellín 53.5% (n = 23), followed by other parts of Antioquia 34.9% (n = 15). The contributory scheme was the most frequent (62.8%, n = 27).
The most common reason for consultation was breast mass, with 97.6% (n = 42); the mean size was 8.6 cm (SD: 4.6. Range 2.1 to 20); the mean time from the appearance of symptoms to the consultation was 4.1 months (SD 3.9. Range 1 to 16 months).
In all cases (n = 43) a palpable mass was found on physical examination, and it was a single lesion. The involvement was in the right breast in 22 (51.2%) and left in 20 (46.5%) cases, and the most frequent location was central and in the upper external quadrant with 41.9% each (n = 18). In 3 cases (7%), axillary adenopathy was palpated on physical examination. 25.5% (n = 11) had a family history of breast cancer.
The paraclinical studies performed included breast ultrasound in 21 cases (48.8%), the most common finding being the nodule 95.2% (n = 20). The BIRADS (Breast Imaging Reporting and Data System) classification with category 4 it was the most frequent, with 76.2% (n = 16), and category 5 with 4.7% (n = 1). The most frequent histological types were benign and borderline with 34.9 (n = 15) each. The paraclinical characteristics are shown in Table 1.
The most frequently performed surgery was quadrantectomy, in 55.8% (n = 24). Negative margins were obtained in 81.4% (n = 35). In 7 cases (16.3%) there were positive margins, and in 6 cases margin expansion was carried out. The mean follow-up was 60.3 months (SD = 57.4. Range 4 to 275). In no case was adjuvant chemotherapy used. Treatment and follow-up characteristics are shown in Table 2.
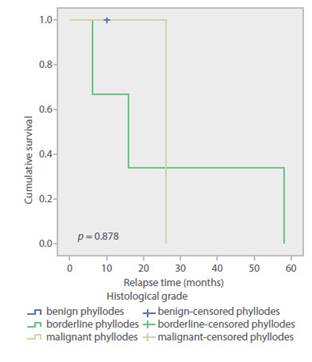
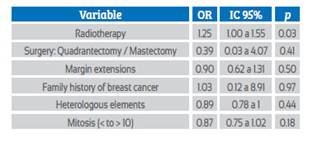
Analysis was carried out regarding the risk of recurrence and the types of treatment and risk factors. The PTs taken to radiotherapy had an OR 1.25 (95% CI 1.0 to 1.55; p = 0, 03), possibly because they were tumors with a worse prognosis (Table 3). Disease-free survival was 90.7% (n = 39) and the mean time to relapse, 23.2 months (SD: 20.8. Range 6 to 58). Figure 2 shows the Kaplan Meier curves for disease-free survival according to histological types.
Table 3 bivariate analysis for association between recurrence of phyllodes tumor and the different types of treatment and risk factors.
Discussion
The prevalence of phyllodes tumor in the present study was 1.3%, similar to that published by Lin C et al. 3, 0.9%. Due to the infrequency of this type of tumor, epidemiological data are few. Berstein et al.(5, during a period of 17 years, determined the average annual incidence of 2.1 cases x 106 women.
The mean age in this series was 42 years, with a range between 11 and 85 years, similar to that found by Orea et al.(7, average age 40 years and range between 14 and 82 years. A quarter, in the present study, had a family history of breast cancer. However, it is currently considered that there are no predisposing etiological factors19.
This type of tumor has a growth pattern that can be accelerated, achieving large sizes, with an average of 4 to 7 cm, at which point they are perceived on self-examination19. In the present series, the average size was 8.6 cm, similar to that found by Galvis et al.(20 in the Hispanic population, with a mean size of 7.7 cm. Palpable lymphadenopathy occurs in 44% of patients with PT, being mostly inflammatory21. In the present study, 7% presented adenopathy on physical examination, without histological confirmation of involvement in any of the cases.
The initial diagnostic approach requires mammography and ultrasound. PT has the appearance of a nodule with well-defined contours, macrolobed, similar to a fibroadenoma22. It is not possible to discriminate histological types through imaging findings that allow their differentiation23. In published case series, it has been found that magnetic resonance evaluation could distinguish PTs from borderline and malignant histology, identifying septa with low internal signal and sediment-like patterns in T2 sequences24.
When clinical suspicion persists, despite a biopsy showing a fibroadenoma or with equivocal pathology for PT, an excisional biopsy is recommended for the complete study of the specimen25.
According to histological parameters, PT is classified as benign, borderline, and malignant. Approximately 52% are benign, 13% borderline, and 35% malignant13. In this study, it was found that the benign type was lower than that published, and corresponded to 34.9%, while the malignant type was 30.2%, similar to that found by Macdonald et al.13). However, when compared with a study in a Latin American population, the benign type was 33.7% and the borderline was 31.1%26), similar to that found in the present study.
The main treatment is surgical, with the aim of reducing local disease relapse, disease-free survival, and overall survival27. Surgery can range from conservative in benign tumors to performing mastectomy in tumors with poor breast-tumor ratio.
Surgical treatment requires resection with negative margins of at least 10 millimeters. Total mastectomy is recommended if negative margins cannot be achieved. In the present study, conservative surgery was performed in 55.8% of tumors, similar to what was reported in a series of 77 cases, with 50.6% conservative surgery26.
The risk of recurrence with wide local resection generates local relapse rates of 8% for benign PT and 21-36% for borderline and malignant tumors28. In the present study, local relapses were 9.3%, similar to that published in other Latin American series, between 4.3% and 7.8%20,26).
In a retrospective review of 83 articles, which included 5 530 patients, carried out by the Milan Oncological Institute, the majority were treated with quadrantectomy (95%), with negative surgical margins. The 5-year local relapse rate for benign PT was 8%(28). For borderline TF, the recurrence rate was 14-17%, and changes when the margins are wide from 14 to 36.6% and for mastectomy 8.3%29).
In a series of cases carried out at the Royal Marsden, with 322 women with malignant phyllodes tumor who underwent surgery, the predictive factors of local and systemic recurrence at 9 years were analyzed, finding that positive surgical margins and the size of the lesions are strong predictors of recurrence, with the probability of recurrence being seven times higher in tumors >10 cm (HR = 11.1, p = 0.006) and four times higher when negative margins of disease are not guaranteed (HR = 4.9, p = 0.008)29).
Other factors, such as cellularity, number of mitoses, stromal growth, and type of surgery performed, have not been statistically significant in predicting the risk of local recurrence29. In the present study, no statistically significant difference was found for the risk of recurrence according to the type of surgery, quadrantectomy versus mastectomy, when performing enlargements, family history of breast cancer, presence or absence of heterologous elements, and mitosis less than or greater than 10.
The benefit of radiotherapy in the treatment of PT is uncertain, and is based on small cohorts29. Its use is based on the results obtained in patients with soft tissue sarcomas29. Chaney et al. report the use of adjuvant radiotherapy (doses of 50 to 60 Gy) in malignant tumors with negative margins <0.5 cm, in tumors >10 cm and after resections for local relapses30. They report local recurrence rates of 9 to 13%, being higher when the margins are positive, showing the usefulness of radiotherapy when negative margins are obtained. In the present study, negative margins were achieved in 95.3% of the cases and adjuvant radiotherapy was used in 46.5%, finding that the group with radiotherapy had a higher risk of recurrence, possibly due to being a selected group of patients with more aggressive histology or larger tumor size.
Gnerlich JL et al. analyzed 3 120 patients with malignant PT treated with surgery and radiotherapy in 14.3% of cases, showing general recurrence rates of 14% and local recurrence of 5.9%, with no impact on disease-free survival or overall survival31. The recurrence-free period improved from 14 to 25 months for conservative surgery, but not for mastectomy (p = 0.006)31).
Spitaleri G et al. found no significant difference with the use of adjuvant radiotherapy in distant and global metastasis-free survival28.
Limitations of the present study include its retrospective nature and the loss of data from diagnostic imaging studies.
In conclusion, the phyllodes tumor is a rare neoplasm, with no clear etiology, that affects women in the fourth decade of life. It presents as a large, unilateral, rapidly growing mass that can achieve large dimensions at the time of diagnosis. Core needle diagnosis makes it difficult to differentiate from fibroadenoma. A greater predominance of the borderline histological type is found in the Hispanic population. The risk of recurrence is significant, and surgery with wide negative margins and radiotherapy are required in selected cases.
REFERENCES
1. Tan PH, Thike AA, Tan WJ, Myint Thu MM, Busmanis I, Li H, et al. Predicting clinical behaviour of breast phyllodes tumours: a nomogram based on histological criteria and surgical margins. J Clin Pathol. 2012;65(1):69-76. doi:10.1136/jclinpath-2011-200368 [ Links ]
2. Muller J. Uber den feinern Bau und die Form der krankhaften Geschwulste: in zwei Lieferungen. Berlin: G Reimer;1838. [ Links ]
3. Lin C-C, Chang H-W, Lin C-Y, Chiu C-F, Yeh S-P. The clinical features and prognosis of phyllodes tumors: a single institution experience in Taiwan. Int J Clin Oncol. 2013;18(4):61420. doi:10.1007/s10147-012-0442-4 [ Links ]
4. Tan P-H, Jayabaskar T, Chuah K-L, Lee H-Y, Tan Y, Hilmy M, et al. Phyllodes tumors of the breast: The role of pathologic parameters. Am J Clin Pathol. 2005;123(4):529-40. doi:10.1309/U6DVBFM81MLJC1FN [ Links ]
5. Bernstein L, Deapen D, Ross RK. The descriptive epidemiology of malignant cystosarcoma phyllodes tumors of the breast. Cancer. 1993;71(10):3020-4. doi:10.1002/1097-0142( 19930515)71:10<3020::aid-cncr2820711022>3.0.co;2-g. [ Links ]
6. Birch JM, Alston RD, McNally RJ, Evans AM, Eden OB, Varley JM. Relative frequency and morphology of cancers in carriers of germline TP53 mutations. Oncogene. 2001;20(34):4621-8. doi:10.1038/sj.onc.1204621 [ Links ]
7. Orea-Estudillo D, Jaimes-López L, Bernal-Cano J. Tumor phyllodes en un paciente pediátrico. Informe de un caso y revisión de la literatura [Phyllodes tumor in a pediatric patient. Case report and literature review]. Cir Cir. 2008;76(2):165-8. [ Links ]
8. Rosai J. In: Rosai J Ackerman V, editors. Surgical Pathology. 10a edition. Philadelphia: Elsevier Mosby; 2010:1659-770. [ Links ]
9. Tavassoli F. Pathology and genetics. Tumours of the breast and female genital tract. No. 4. IARC; 2003. [ Links ]
10. Kumar V, Abbes AK, Aster JC. In: Robbins S, Cotran C, editors. Pathologic basis of disease, 8a edition. Philadelphia: Saunders Elsevier; 2011:1065-95. [ Links ]
11. Meyer JE. Large-core needle biopsy of nonpalpable breast lesions. JAMA. 1999;281(17):1638. doi:10.1001/jama.281.17.1638 [ Links ]
12. Lee AHS, Hodi Z, Ellis IO, Elston CW. Histological features useful in the distinction of phyllodes tumour and fibroadenoma on needle core biopsy of the breast. Histopathology. 2007;51(3):336-44. doi:10.1111/j.1365-2559.2007.02786.x [ Links ]
13. Macdonald OK, Lee CM, Tward JD, Chappel CD, Gaffney DK. Malignant phyllodes tumor of the female breast: Association of primary therapy with cause-specific survival from the Surveillance, Epidemiology, and End Results (SEER) program. Cancer. 2006;107(9):2127-33. doi:10.1002/cncr.22228 [ Links ]
14. de Roos WK, Kaye P, Dent DM. Factors leading to local recurrence or death after surgical resection of phyllodes tumours of the breast: Phyllodes tumours of the breast. Br J Surg. 1999;86(3):396-9. doi:10.1046/j.1365-2168.1999.01035.x [ Links ]
15. Adesoye T, Neuman HB, Wilke LG, Schumacher JR, Steiman J, Greenberg CC. Current trends in the management of phyllodes tumors of the breast. Ann Surg Oncol. 2016;23(10):3199205. doi:10.1245/s10434-016-5314-0 [ Links ]
16. Park HJ, Ryu HS, Kim K, Shin KH, Han W, Noh D-Y. Risk factors for recurrence of malignant phyllodes tumors of the breast. In Vivo. 2019;33(1):263-9. doi:10.21873/invivo.11470 [ Links ]
17. Barth RJ, Wells WA, Mitchell SE, Cole BF. A prospective, multi-institutional study of adjuvant radiotherapy after resection of malignant phyllodes tumors. Ann Surg Oncol. 2009;16(8):2288-94. doi:10.1245/s10434-009-0489-2 [ Links ]
18. Morales-Vásquez F, Gonzalez-Angulo AM, Broglio K, Lopez-Basave Hn, Gallardo D, Hortobagyi GN, et al. Adjuvant chemotherapy with doxorubicin and dacarbazine has no effect in recurrence-free survival of malignant phyllodes tumors of the breast: Chemotherapy for phyllodes tumors. Breast J. 2007;13(6):551-6. doi:10.1111/j.1524-4741.2007.00510.x [ Links ]
19. Corso D, Contreras D, Ángel J, Guzmán L, Díaz S, García O y col. Tumor filoide. Estado del arte. Rev Colomb Cancerol. 2016;20(2):79-86. doi:10.1016/j.rccan.2015.10.001 [ Links ]
20. Galvis LR, Bolívar E, Ruiz A, Cáceres A, Agüero A. Factores clínico histológicos y pronóstico del tumor phyllodes de mama: análisis de datos de nuestra institución. Rev venez oncol. 2014;26(1):9-15. [ Links ]
21. Rodríguez Rojas A, Gutierrez R. Revista Colombiana de Cirugía. 1994;9(3):157-62. [ Links ]
22. Calhoun KE, Thomas J, Nam Kim J, Lehman CD, Anderson BO. Phyllodes tumors. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast. 4th Edition. Lippincott Williams & Wilkins; 2010:782-92. [ Links ]
23. Ibáñez G, Marambio A, Jans J, Gamboa J, Adonis P, Trewhela R, y col. Tumor filoides de la mama. Rev Chil Cir. 2010;62(2):119-24. [ Links ]
24. Tan H, Zhang S, Liu H, Peng W, Li R, Gu Y, et al. Imaging findings in phyllodes tumors of the breast. Eur J Radiol. 2012;81(1):e62-9. [ Links ]
25. Dillon MF, Quinn CM, McDermott EW, O'Doherty A, O'Higgins N, Hill AD. Needle core biopsy in the diagnosis of phyllodes neoplasm. Surgery. 2006;140(5):779-84. [ Links ]
26. Ossa CA, Herazo F, Gil M, Echeverri C, Angel G, Borrero M, Madrid J, Jaramillo R. Phyllodes tumor of the breast: a clinic-pathologic study of 77 cases in a Hispanic cohort. Colomb Med (Cali). 2015;46(3):104-8. [ Links ]
27. Guillot E, Couturaud B, Reyal F, Curnier A, Ravinet J, Laé M, et al. Management of phyllodes breast tumors. Breast J. 2011;17(2):129-37. [ Links ]
28. Spitaleri G, Toesca A, Botteri E, Bottiglieri L, Rotmensz N, Boselli S, et al. Breast phyllodes tumor: A review of literature and a single center retrospective series analysis. Crit Rev Oncol Hematol. 2013;88(2):427-36. doi:10.1016/j.critrevonc.2013.06.005 [ Links ]
29. Kapiris I, Nasiri N, A'Hern R, Healy V, Gui GP. Outcome and predictive factors of local recurrence and distant metastases following primary surgical treatment of high-grade malignant phyllodes tumours of the breast. Eur J Surg Oncol. 2001;27(8):723-30. [ Links ]
30. Chaney AW, Pollack A, McNeese MD, Zagars GK, Pisters PW, Pollock RE, y col. Primary treatment of cystosarcoma phyllodes of the breast. Cancer. 2000;89(7):1502-11. [ Links ]
31. Gnerlich JL, Williams RT, Yao K, Jaskowiak N, Kulkarni SA. Utilization of radiotherapy for malignant phyllodes tumors: analysis of the National Cancer Data Base, 1998-2009. Ann Surg Oncol. 2014;21(4):1222-30. [ Links ]
Received: August 31, 2020; Accepted: November 03, 2020











 texto en
texto en