Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.67 no.1 Lima Jan-Mar 2021
http://dx.doi.org/10.31403/rpgo.v67i2309
Case Report
Prenatal diagnosis of placental mesenchymal dysplasia
1. Obstetrics and Gynecology Service, Central Hospital "Dr. Urquinaona , Maracaibo, Zulia state, Venezuela
Placental mesenchymal dysplasia is a rare and benign placental abnormality. It is characterized by placentomegaly with multiple cystic lesions of the villous tree and vascular abnormalities along with increased maternal serum alpha-fetoprotein concentrations. It is an unknown clinical entity and can be confused with gestational trophoblastic disease due to the similar ultrasound findings in both entities; but the management and the perinatal outcome are different. It should be included in the differential diagnosis of ultrasound findings showing a normal-appearing fetus and a placenta with cystic lesions. However, placental mesenchymal dysplasia is associated with complications such as intrauterine growth restriction and intrauterine fetal death. Due to the high number of complications and associated pathologies, it is necessary to increase ultrasound evaluations to reduce fetal morbidity and mortality. A case of prenatal diagnosis of placental mesenchymal dysplasia with normal fetus is presented.
Key words: Placental diseases; Ultrasound; Prenatal diagnosis
Introduction
Placental mesenchymal dysplasia (PMD) is a rare and benign placental vascular developmental anomaly. It is characterized by placentomegaly with multiple cystic lesions of the truncal villi and vascular anomalies1. It is usually misdiagnosed, as it is poorly understood and may be confused with gestational trophoblastic disease due to similar ultrasound findings. Unlike molar pregnancies, which are characterized by a malformed or absent fetus, pregnancies with PMD last until the third trimester without significant maternal morbidity2.
Most pregnant women with PMD have intrauterine growth restriction, intrauterine fetal death, and other congenital anomalies. Termination of pregnancy is usually unnecessary when there is certainty of the condition. Pathological examination of the placenta allows definitive diagnosis2,3. A case of prenatal diagnosis of placental mesenchymal dysplasia is presented.
Presentation of the case
The case was a 30-year-old primigravid patient with a 23-week pregnancy, who was referred to the high-risk prenatal clinic due to placental morphological alterations in the routine ultrasound. The pregnancy was spontaneous and had been uncomplicated up to that time. The patient denied genital bleeding or abdominal pain. Ultrasonography at 8, 11 and 15 weeks showed no abnormalities of the fetus and/or placenta. First trimester aneuploidy screening revealed a 1:780 risk for Down syndrome. She denied alcohol, tobacco or recreational drug use and significant personal or family history.
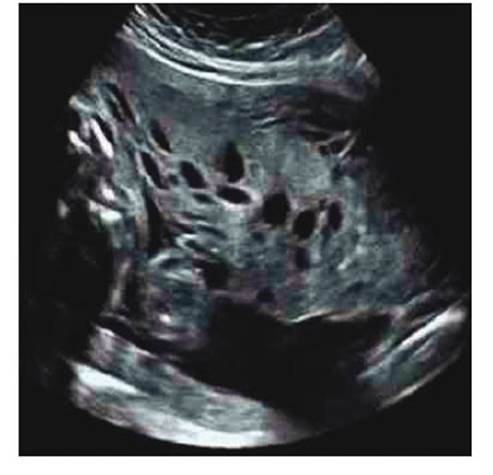
Ultrasonography showed single 23-week female fetus by fetal biometry, with no obvious structural abnormalities, with normal amniotic fluid volume. But she was in the 10th percentile by fetal growth curve for gestational age. The placenta was slightly thickened, with presence of multiple anechoic lacunar images within the parenchyma (Figure 1). Color Doppler showed no internal blood flow in the lesions. In view of the ultrasound and Doppler findings, the possibility of partial molar pregnancy, PMD or Beckwith Wiedemann syndrome was considered, and it was decided to perform amniocentesis, the result of which was normal fetal karyotype (46,XX) and multiple ligand-dependent probe amplification analysis was negative for the syndrome.
Routine hematologic and biochemical test results were within normal limits. Human chorionic gonadotropin concentrations were normal, but serum alpha-fetoprotein concentrations (193.48 ng/mL) were elevated. The case was discussed with the patient and she was informed of the risks of complications. Conservative management was decided.
During the follow-up ultrasound evaluation at 26 weeks, accentuation of intrauterine growth retardation (3rd percentile for gestational age) was evident, with no evidence of fetal morphological alterations and normal amniotic fluid volume. Fetal Doppler evaluation was within normal limits, showing absence of vascular flow within the vesicular lesions. Due to the findings, corticosteroid treatment was initiated to accelerate fetal lung maturation. Subsequently, the patient attended the emergency room due to decreased fetal movements, confirming the diagnosis of intrauterine fetal death. Uterine evacuation was performed with misoprostol, obtaining a 1 025 gram female stillbirth. The delivery was spontaneous, obtaining a hypertrophic and irregular placenta of 430 grams, with an umbilical cord of normal length and diameter.
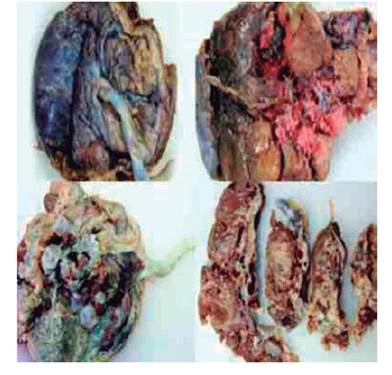
Pathological examination of the placenta showed that on the maternal side the cotyledons were intact, with an ischemic appearance and covered with fibrin. The fetal surface showed areas of normal appearance along with numerous clusters of grape-shaped fluid-filled cavities measuring up to 2 centimeters in diameter. The umbilical cord showed eccentric insertion. Tissue section showed that the intervillous space had cystic cavities 1 to 2 centimeters in diameter with dysplastic villi (Figure 2).
Microscopic evaluation showed large, edematous focal villi with central myxoid-lax stroma and large central cistern. The vessels were prominent thick-walled with fibromuscular hyperplasia, marked luminal stenosis and intimal sclerosis located peripherally with some of them obstructed and lined with hypoplastic trophoblastic cells without atypia or mitosis. There were also normal-sized chorionic villi interspersed with maturation consistent with gestational age. There was no evidence of hyperplasia, stromal cell inclusions, or other features of trophoblastic proliferation around the abnormal villi. Immunohistochemical examination showed strong positivity for desmin and vimentin in the stromal cells of normal and abnormal villi, whereas smooth muscle actin was present in normal villous cells (Figure 3). Cells lining the cisterna were negative for D2-40 and CD34, but stained with vimentin. There was also evidence of detectable low Ki-67 expression in any of the tissues. The final diagnosis was MPD.
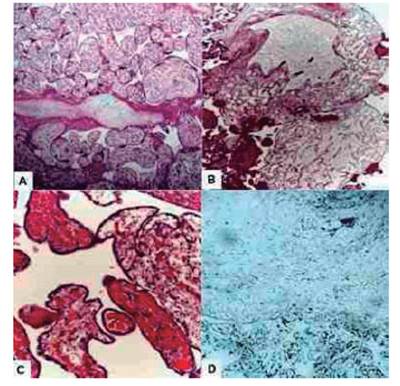
Figure 3 Histological image of placental mesenchymal dysplasia. (A) Placental villi with chorioangiosis. (B) Multiple vesicles within areas of placental tissue. (C) Dilated villi with absence of proliferative trophoblastic tissues with multiple dilated capillaries. (D) Strong, positive, and generalized desmin immunostaining.
The patient was discharged on the third day. Alpha-fetoprotein concentrations decreased significantly at 7 days and remained within normal limits for 6 months after delivery.
Discussion
MPD is characterized by the presence of a normal appearing fetus together with a thickened placenta with multiple lacunar or anechoic cystic lesions, with low or absent vascular flow on Doppler evaluation and widely distributed edematous villi4. Its approximate incidence is 0.02% of pregnancies and a female to male sex ratio of 3.6:11. Some synonyms used for this condition are placentomegaly with massive hydrops of placental stalk villi, hairy mesenchymal stalk hyperplasia and pseudo-lacunar mole5-7.
The etiology of PMD is not clearly defined. Some theories propose that it is a congenital malformation of the extraembryonic mesoderm4. This is based on evidence of mesenchymal hyperplasia of the truncal villi along with other placen-tal proliferative disorders and dilatation of the chorionic vessels. Hypoxia/hypoperfusion of un known etiology appears to stimulate fibroblasts to overproduce connective tissue, increasing vascular endothelial growth factor production leading to angiogenesis and vascular malformations. Both malformations and circulatory imbalance contribute to the formation of cisternae within the villi8. Another theory showed that placental vesicular lesions may be caused by abnormal lymphangiogenesis9. However, several findings confirm that stromal cells in abnormal villi stop differentiating beyond the fibroblast stage10.
Alteration of chromosome 11p15.5, together with mutations of the CDKN1C (p57kip2), H19, IGF-II and KVLQT genes are also commonly associated with the occurrence of MPD. The p57kip2 gene encodes a cyclin-dependent kinase inhibitor. Alteration of chromosome 11p15.5 encodes a specific insulin-like growth factor in the fetus that can lead to placental tissue overgrowth. It has also been proposed that modifications on the X chromosome may contribute to the development of dysplasia, but the exact underlying mechanism has not yet been established4.
The clinical symptoms of PMD are inconspicuous. Most cases are diagnosed by routine prenatal ultrasonography in early pregnancy. The distinguishing feature is the finding of increased serum concentrations of maternal alpha-fetoprotein. Increased transfer surface area, as a consequence of increased placental volume, and abnormal thin-walled vessels within the truncal villi are central to the abnormally increased passage of alpha-fetoprotein into the maternal circulation11. On the other hand, human chorionic gonadotropin concentrations may remain normal or increase slightly during pregnancy and return to normal after delivery5.
Ultrasonographic findings reveal thickened placenta with multicystic appearance without vascular flow within the lesions. These findings vary with gestational age. Cystic changes and chorionic vascular malformations appear in approximately 70% of cases at 13 to 20 weeks of pregnancy. Only in rare cases are they detected before 13 weeks. On the other hand, 90% of cases present placenta with dilated chorionic vessels in the third trimester that can hardly be observed before 25 weeks of gestation3. In these cases, it is necessary to perform a detailed evaluation to rule out associated fetal anomalies, mainly findings consistent with Beckwith-Wiedemann syndrome, which has been described in 20% of PMD cases1. Doppler evaluation during the first trimester shows no blood flow in the placental cystic spaces. However, during the third trimester, there is evidence of large vascular areas with turbulent arterial or venous blood flow, mainly below the chorionic plate. These changes are due to progressive dilatation of the chorionic arteries and veins, which become aneurysmal. Low or absent venous signals may be associated with the condition during the first two trimesters of pregnancy12.
The diagnosis of MPD should be confirmed by pathologic evaluation of the placenta. The characteristic histologic findings are edematous, markedly enlarged truncal villi with cisternae without trophoblastic proliferation. The vessel walls with fibromuscular hyperplasia are thickened, but the terminal villi show normal architecture, which is the distinguishing feature of hydatidiform mole, together with the fetal karyotype10,13.
The main differential diagnoses by ultrasound and pathological anatomy are partial hydatidiform mole, twin gestation with complete mole or confined placental mosaicism. It is very important to distinguish PMD from partial or embryonal mole. In this pathology there are two types of conditions associated with the fetus: a dyspermic triploid egg with partial mole and true twin pregnancy. In the first case, the fetus is usually malformed and not viable. In the second case, the fetus is chromosomally normal, with a 40% chance of live birth. The histological features for the diagnosis of partial hydatidiform mole are: focal hyperplasia of the syncytiotrophoblast, focal edema of the villi with cavity formation, fenestrated outline of the villi, trophoblastic inclusions within the stroma, vessels in the villi with fetal erythrocytes, and presence of embryo or fetus and fetal membranes14. Confined placental mosaicism can be determined by fetal and placental karyotyping4. Other differential diagnoses include multiple chorioangiomas, multiple subchorionic cysts and spontaneous abortion with hydropic changes.
PMD is associated with complications such as intrauterine growth restriction of the fetus and intrauterine fetal death2. The mechanism by which they occur is unknown and may be heterogeneous. Inadequate fetal blood circulation due to vascular malformations, chronic hypoxia secondary to thrombosis of the blood vessels of the truncal villi and reduced functional capacity of the villi are considered potential causes. Umbilical cord anomalies or thrombosis of the chorionic lacunar spaces may also play a role in the development of complications. Hemorrhage secondary to rupture of fragile and dilated chorionic vessels may lead to intrauterine fetal death8. Fetal anemia and thrombocytopenia may also occur, presumably secondary to the microangiopathic process occurring in the abnormal placental vasculature15 ,16) .
PMD is a benign disorder and termination of pregnancy is not necessary, so only intensive clinical follow-up is required. Pregnant women with suspicious ultrasound findings should be monitored more frequently, due to the increased risk of adverse perinatal outcome. During the third trimester, it is advisable to intensify monitoring of fetal well-being1,12.
Conclusion
PMD is a rare vascular anomaly characterized by placentomegaly with ultrasonographic findings that probably suggest the possibility of hydatidiform mole with a coexisting fetus. Management of these cases is conservative and should be differentiated from molar pregnancy, as termination of pregnancy is not necessary. Due to the high number of complications and associated pathologies, it is necessary to increase ultrasound evaluations to decrease fetal morbidity and mortality.
REFERENCES
1. Psarris A, Sindos M, Kourtis P, Pampanos A, Antsaklis P, Theodora M, et al. Placental mesenchymal dysplasia: Ultrasound characteristics and diagnostic pitfalls. Ultrasound Int Open. 2020;6(1):E2-E3. doi: 10.1055/a-1180-9571 [ Links ]
2. Mittal D, Anand R, Sisodia N, Singh S, Biswas R. Placental mesenchymal dysplasia: What every radiologist needs to know. Indian J Radiol Imaging. 2017;27(1):62-4. doi: 10.4103/0971-3026.202949 [ Links ]
3. Nayeri UA, West AB, Grossetta Nardini HK, Copel JA, Sfakianaki AK. Systematic review of sonographic findings of placental mesenchymal dysplasia and subsequent pregnancy outcome. Ultrasound Obstet Gynecol. 2013;41(4):366-74. doi: 10.1002/uog.12359 [ Links ]
4. Pawoo N, Heller DS. Placental mesenchymal dysplasia. Arch Pathol Lab Med. 2014;138(9):1247-9. doi: 10.5858/arpa.2013-0399-RS [ Links ]
5. Doroftei B, Neculai-Valeanu S, Simionescu G, Grab D, Plopa N, Anton E, et al. A case report of placental mesenchymal dysplasia: A rare case of a genetically normal fetus with severe intrauterine growth restriction. Medicine (Baltimore). 2019;98(8):e14554. doi: 10.1097/MD.0000000000014554 [ Links ]
6. Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018;218(2S):S745-S761. doi: 10.1016/j.ajog.2017.11.577 [ Links ]
7. Kuwabara Y, Yonezawa M, Kubota Y, Ichikawa T, Ohashi R, Takeshita T. Unique clinical and histological features of placental mesenchymal dysplasia complicated by severe preeclampsia in the midtrimester. AJP Rep. 2020;10(1):e113-e117. doi: 10.1055/s-0040-1709186 [ Links ]
8. Francis B, Hallam L, Kecskes Z, Ellwood D, Croaker D, Kent A. Placental mesenchymal dysplasia associated with hepatic mesenchymal hamartoma in the newborn. Pediatr Dev Pathol. 2007;10(1):50-4. doi: 10.2350/06-03-0066.1 [ Links ]
9. Ulker V, Aslan H, Gedikbasi A, Yararbas K, Yildirim G, Yavuz E. Placental mesenchymal dysplasia: a rare clinicopathologic entity confused with molar pregnancy. J Obstet Gynaecol. 2013;33(3):246-9. doi: 10.3109/01443615.2012.745491 [ Links ]
10. Kim B, Hyeon J, Lee M, Hwang H, Shin Y, Choi SJ, et al. Placental mesenchymal dysplasia with fetal gastroschisis. J Pathol Transl Med. 2015;49(1):71-4. doi: 10.4132/jptm.2014.12.14 [ Links ]
11. Guenot C, Kingdom J, De Rham M, Osterheld M, Keating S, Vial Y, et al. Placental mesenchymal dysplasia: An underdiagnosed placental pathology with various clinical outcomes. Eur J Obstet Gynecol Reprod Biol. 2019;234:155-64. doi: 10.1016/j.ejogrb.2019.01.014 [ Links ]
12. Ohira S, Ookubo N, Tanaka K, Takatsu A, Kobara H, Kikuchi N, et al. Placental mesenchymal dysplasia: chronological observation of placental images during gestation and review of the literature. Gynecol Obstet Invest. 2013;75(4):217-23. doi: 10.1159/00035066 [ Links ]
13. Linn RL, Minturn L, Yee LM, Maniar K, Zhang Y, Fritsch MK, et al. Placental mesenchymal dysplasia without fetal development in a twin gestation: a case report and review of the spectrum of androgenetic biparental mosaicism. Pediatr Dev Pathol. 2015;18(2):146-54. doi: 10.2350/14-12-1583-CR.1 [ Links ]
14. Juárez-Azpilcueta A, Islas-Domínguez L, Durán-Padilla M. Mola hidatidiforme parcial con feto vivo del segundo trimestre. Rev Chil Obstet Ginecol. 2010;75(2):137-9. doi: 10.4067/S0717-75262010000200011 [ Links ]
15. Li H, Li L, Tang X, Yang F, Yang KX. Placental mesenchymal dysplasia: a case of a normal-appearing fetus with intrauterine growth restriction. Int J Clin Exp Pathol. 2014;7(8):5302- 7. PMID: 25197414 16. Ishikawa S, Morikawa M, Umazume T, Yamada T, Kanno H, Takakuwa E, et al. Anemia in a neonate with placental mesenchymal dysplasia. Clin Case Rep. 2016;4(5):463-5. doi: 10.1002/ccr3.543 [ Links ]
Declaration of ethical aspects
Acknowledgment of authorship: All authors declare that have made contributions to idea, study design, data collection, data analysis and interpretation, critical review of intellectual content and final approval of manuscript that we are sending.
Ethical responsibilities: Protection of people. Authors declare that procedures followed were in accordance with ethical standards of responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Confidentiality of data: The authors declare that we have followed protocols on the publication of patient data.
Right to privacy and informed consent: The authors have obtained the informed consent of the patients and / or subjects referred to in the article. This document is in possession of the corresponding author.
Financing: The authors certify that we have not received financial support, equipment, work personnel or in kind from people, public and / or private institutions to carry out the study.











 text in
text in