Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.1 Lima ene./mar. 2022 Epub 24-Feb-2022
http://dx.doi.org/10.31403/rpgo.v68i2402
Original Articles
Qualitative human beta chorionic gonadotropin for the diagnosis of premature rupture of membranes in Honduras
1 Medical Specialist in Gynecology and Obstetrics, Dr. Mario Catarino Rivas Hospital, San Pedro Sula, Honduras.
2 Department of Epidemiology, Hospital Dr. Mario Catarino Rivas, San Pedro Sula, Honduras.
3 Scientific Association of Medical Students, Universidad Católica de Honduras Campus San Pedro y San Pablo (ASOCEM UNICAH SPSP), San Pedro Sula, Honduras.
4 Medical Specialist in Oncology, MSc in Bioethics, PhD in Public Health, Liga Contra el Cáncer, San Pedro Sula, Honduras.
5 Clinical Oncologist, Chief (Clinical Oncology) Liga contra el Cáncer, Honduras
Objective
: To analyze qualitative human beta chorionic gonadotropin (β-hCG) as a diagnostic method for premature rupture of membranes (PROM).
Methods
: Prospective case-control study, with a non-probabilistic sample by convenience, of 90 women between 24 and 40 weeks of gestation divided into two groups: study group (45 patients with clinical diagnosis of PROM) and control group (45 patients hospitalized without PROM). Vaginal lavage or aspirate was performed to qualitatively determine β-hCG in commercial β-hCG measurement kits with threshold of 25 mUI/mL as well as nitrazine paper test.
Results
: The sensitivity, specificity, positive predictive value, and negative predictive value for the β-hCG-25 test were 77.8% (95% CI, 63.7-87.5), 82.2% (95% CI, 68.7-90.7), 81.4%, and 78.7%, respectively. Diagnostic accuracy was 80.0% (0.6 Landis & Koch kappa index) versus 75.6% for nitrazine pH-metry.
Conclusions
: The qualitative β-hCG test showed a representative diagnostic value and can corroborate the early diagnosis of PROM, recommending it as a simple, rapid, accessible and low-cost test.
Key words: Fetal membranes; premature rupture; Chorionic gonadotropin; beta subunit; human; Amniotic fluid; Pregnancy complications
INTRODUCTION
Premature rupture of the ovular membranes (PROM) is defined as the loss of continuity of the chorioamniotic membrane before the onset of labor at term or preterm (PROM) before 37 weeks1,2. Its incidence is 10% of all pregnancies(3,4). Its etiology is multifactorial and can be affected by biochemical, physiological, pathological and environmental factors5,6. PROM leads to maternal and fetal risks in approximately 8% of cases7.
The clinical picture is characterized by amniotic fluid leakage without contractions1. The diagnosis of spontaneous rupture of the membranes is made by maternal history followed by sterile speculum examination demonstrating frank fluid in the cervical canal. This diagnoses 90% of cases1,8,9. When a pool of amniotic fluid is not clearly seen, IGFBP1 or PAMG-1 testing should be considered, if available12. Of the diagnostic laboratory studies, the gold standard for confirming PROM is to inject indigo carmine into the amniotic sac during amniocentesis and then evaluation for any blue fluid visibly leaking through the cervix or pooling in the vaginal vault, an invasive method not recommended for all patients13. Traditionally, pH-metry with nitrazine test and the fern test to visualize the characteristic 'fern-leaf' shape of sodium chloride in amniotic fluid have been used as diagnostic adjuncts in uncertain cases and have a sensitivity of 4281%7,8). They are limited by their number of false negatives and positives. After 48 hours of RPM latency, the nitrazine test and the fern test show 9.4% and 13-30% false negatives, respectively7. Other methods such as those mentioned above are also dependent on successful sample collection1,10. No studies have been identified that specifically address ultrasonography to determine amniotic fluid volume in women presenting with suspected PROM. Ultrasound examination showing oligohydramnios may be useful to support the clinical diagnosis of PROM12.
The diagnosis of PROM is difficult when there are long periods of latency or discrete rupture, due to a scarce or intermittent discharge of amniotic fluid1,7,8. Its diagnosis is crucial for treatment decisions, since 75% of term gestations will go into spontaneous labor within 24 hours and 48% of preterm gestations will go into spontaneous labor within 72 hours2,11.
Despite the existence of alternative non-invasive diagnostic methods, such as those mentioned above, the search has been extended to a lower cost and easily accessible test with varied biochemical agents to be performed in amniotic fluid 7,8,14,15. Thus, agents such as β-human chorionic gonadotropin hormone subunit β (β-hCG), a glycoprotein produced by the syncytiotrophoblast present at levels of 2,000 to 70,000 mIU/ mL in amniotic fluid, serum, and maternal urine, have been studied7,9,14,16. The normal concentration of β-hCG in amniotic fluid in the first trimester may be 37.9 mIU, in the second trimester 9.5 mIU, and in the third trimester 6.3 mIU. In PROM, the mean concentration is 342.28 mIU/mL. The cut-off point for the diagnosis of PROM in the second and third trimesters is 17.1 mIU/mL17.
Quantitative analysis of β-hCG for PROM is not practical1,18,19, but qualitatively the results suggest acceptable ranges of diagnostic sensitivity and specificity, showing that it can potentially be a simpler, easier, faster and cheaper method8,14,20,21. This study aims to qualitatively analyze β-hCG in vaginal lavage as a confirmatory diagnostic tool in pregnant women with PROM, with a cut-off level of 25 mIU/mL.
Methods
An analytical observational study, case-control type, was conducted in the Gynecology and Obstetrics Service of the Dr. Mario Catarino Rivas Hospital (HMCR), San Pedro Sula, Honduras, during November 2017 to June 2019. The sampling was non-probabilistic by convenience. Ninety pregnant patients were included, between 24-40 weeks of gestation, divided into two groups. Group A (cases) consisted of 45 patients clinically diagnosed with PROM after observation of fluid in the vagina in the cervical canal by speculoscopy performed by the obstetrician-gynecologist, complemented with an alkaline pH in the nitrazine test. Group B (controls) consisted of 45 hospitalized patients with intact membranes who met the same inclusion and exclusion criteria.
We excluded patients with chronological age less than 13 years and more than 45 years, uterine cervical dilatation greater than 0 cm, or permeable external cervical os with loss of its integrity, absence of cervical mucus plug, threatened or preterm labor, use of vaginal douching, and vaginal bleeding.
A file was designed that included personal data from the clinical history (age), gynecological-obstetric history (gestations, deliveries, cesarean sections, abortions, date of last menstrual period, probable date of delivery), prenatal control (Yes/No) and number of prenatal controls, gestational age by date of last menstrual period or ultrasound of the first trimester of pregnancy, nitrazine strip results and qualitative β-hCG test in vaginal fluid.
The diagnosis of premature rupture of the membrane was established in those patients who had frank leakage of amniotic fluid in the cervical canal by spectroscopy. After signing the informed consent form, the sample was obtained by means of a sterile speculum in the vagina and vaginal aspiration with a 10 mL syringe or vaginal lavage with 1 mL of saline solution, in case the amount of amniotic fluid was scarce. Five drops of the sample were placed in the commercial pregnancy test kit with a threshold of 25 mIU/ mL. The results were noted as positive or negative according to the manufacturer's instructions. The remaining liquid was sprayed onto a nitrazine strip for pH-metry. Both the qualitative β-hCG test and the nitrazine paper test were applied to both groups.
The data were entered into a collation table in the IBM SPSS version 25.0 program. The descriptive analysis of the numerical variables was evaluated with the assumptions of normality and then the categorical variables were described using median and interquartile ranges, analyzing percentages and frequencies. Comparisons between qualitative test results of ß-hCG and nitrazine strips in patients with PROM and the control group were performed using Pearson's chi-square (X2). Diagnostic test analysis was used to obtain the parameters, according to the gold standard (clear evidence of amniotic fluid leakage). The following parameters were chosen sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), diagnostic precision (DP) and kappa index (Landis & Koch criteria); 95% confidence intervals (CI) were calculated for all the above. For analytical statistics, a p <0.05 was considered statistically significant.
Regarding ethical aspects, this study was conducted in accordance with the good clinical practices derived from the International Conference on Harmonization, in addition to complying with all current institutional laws. It had the approval of the institutional ethics committee of the Catholic University of Honduras with # COM-2017-004. All patients gave their signed consent to participate.
Results
The clinical and gynecological characteristics of cases and controls are presented in Table 1. The chronological age of the cases was slightly higher in the controls than in the cases (2.1 years). The difference in gestational age of both groups was 0.5 week. Compared to the controls, there was a higher incidence of term gestation in the cases, as well as of prenatal control and ultrasound.
Table 1 clinical and gynecologic characteristics of patients for comparative testing of qualitative β-hcg as a marker of premature rupture of ovarian membranes according to group a and b.
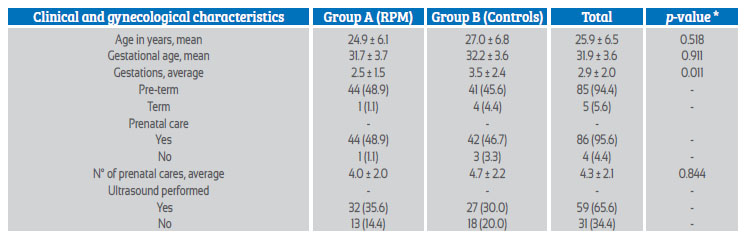
* Levene's test (homogeneity of variances)
The median age of the study was 25 years old [IQR, 20-29], with a range from a minimum age of 18 years to a maximum of 43 years. The median gestational age at the time of the study was 32.1 weeks [IQR, 23.3-24.9]. Of the pregnant women, 94.4% had a preterm pregnancy (85) and 5.6% were at term 5. A total of 95.6% of patients had attended prenatal controls (86), with a median of 4 [IQR, 3-6] controls per patient. The integrity of the fluid in the amniotic sac was evaluated by ultrasound in 65.6% of the patients (59). Table 1.
Qualitative β-hCG of vaginal fluid was positive in 77.8% of patients in group A (35) and 17.8% in group B 8. 22.2% (10) of the tests were false negative (p=<0.001) (Table 2). The nitrazine paper test was positive in 84.4% of patients in group A (38) and 33.3% in the control group 15, resulting in 15.6% false negatives 7 (p=<0.001).
Table 2 distribution of patients in group a (clinical diagnosis of prom) and group b (controls) according to qualitative β-hcg test of vaginal fluid and nitrazine strips.
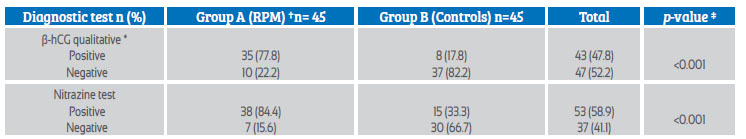
*β-hCG= Qualitative human chorionic gonadotropin beta subunit test with cut-off point of 25 mIU/mL
† PROM = Premature rupture of ovular membranes.
‡ Pearson's chi-square (X2) with significance level of p=0.05
The sensitivity of the qualitative β-hCG test was 77.8% (95% CI, 63.7-87.5), specificity of 82.2% (95% CI, 68.7-90.7), positive predictive value (PPV) of 81.4% (35/43), negative predictive value (NPV) of 78.7% (37/47) and a diagnostic accuracy of 80.0% for RPM (Landis & Koch kappa index 0.6) versus 75.6% for pH-metry in the nitrazine paper test (Table 3).
Table 3 efficacy of nitrazine strips and qualitative measurement of β-hcg relative to the gold standard (clinical evidence of amniotic fluid leakage) for detection of prom.
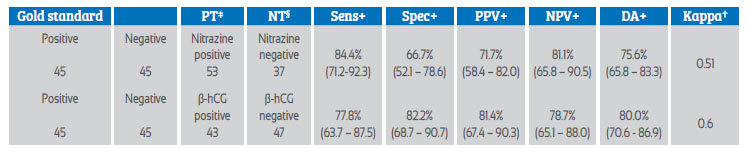
Values in crosses represent 95% confidence intervals. PT‡ = positive test, NT§ = negative test, Sens+ = sensitivity, Spec+ = specificity, PPV+ = positive predictive value, NPV= negative predictive value, DA+ = diagnostic accuracy; Kappa† = kappa interpretation (Landis & Koch criteria): <0.0 poor (poor), 0.01-0.20 slight (slight), 0.21-0.40 fair (fair), 0.41-0.60 moderate (moderate), 0.61-0.80 substantial (substantial), 0.80-1.0 almost perfect (almost perfect).
Discussion
A sensitivity of 77.8%, specificity of 82.2%, PPV of 81.4% and NPV of 80% were found with a cut-off value of 25 mIU/mL. Other studies with the same cutoff value obtained a sensitivity of 44.4%, 87.7% and 93.41%, specificity of 87.1%, 100% and 73.81%, positive predictive value of 66.6%, 100% and 79.44% and negative predictive value of 72.9%, 65% and 91.18%, respectively(7,17,18). Bufalino G et al. (2003), in their study with 120 participants with a cut-off value of 17.1 mIUI/mL, found sensitivity values of 98.3%, specificity of 93.3%, positive predictive value 93.6% and negative predictive value 98.5%22). Kim Y et al. (2005), in another comparative study of 120 patients with a cut-off value of 39.8 mIUI/mL, determined the sensitivity, specificity, positive predictive value and negative predictive value to be 95.5%, 94.7%, 91.3% and 97.3%, respectively(10). Finally, the study with the highest cut-off value (100 mIUI/mL), performed by Urdaneta A et al. (2014), presented values with a sensitivity of 97.0% (95% CI; 92.5-99.1%), specificity of 51.1% (95% CI; 42.3-59.8%), positive predictive value of 66.5% (95% CI; 59.4-73.0%) and negative predictive value of 94.5% in 270 patients8.
A relevant difference has been shown between hCG concentrations in the diagnosis of PROM in the second and third trimester, occurring in proposals of different cutoff values19. The variability in cut-off values, sensitivity, specificity, and predictive values for β-hCG in vaginal fluid could be due to the difference in the number of samples studied and inclusion criteria considered.
Despite being one of the most common pathologies in obstetrics, timely intervention in premature rupture of membranes may be limited by the inability to diagnose it due to the number of false negatives and false positives with traditional diagnostic tools, resulting in an erroneous or delayed diagnosis18. Misdiagnosis leads to unnecessary interventions such as hospitalizations, antibiotic therapy and induction of labor, to be performed only in term PROM1,2. If labor is not induced, 60-70% of them start labor spontaneously within 24 hours and almost 95% will do so in less than 72 h6. On the other hand, under-diagnosis leads to complications such as preterm labor and chorionamnionitis1.
The measurement of qualitative β-hCG is the simplest and most practical tool and can be used in the emergency room of most health facilities23. Among other potential advantages, it has been involved in the prediction of preterm delivery, due to a significantly higher vaginal level in patients undergoing preterm delivery without PROM than in patients with term deliveries10,24,25. Furthermore, obtaining amniotic fluid noninvasively using a commercial β-hCG kit might even allow the evaluation of patients with severe oligohydramnios in whom amniocentesis is not possible, with a reliable detection rate4,10,14.
Consideration should be given to the size of the sample and not generalize these results to national characteristics; and that a market-specific qualitative test of pregnancy is not set, but a cut-off point is established. Even with statistically significant results, we consider that future studies are needed that include a larger sample and the opportunity to consider new variables and correlations to systematically validate the measurement.
In conclusion, the qualitative β-hCG test shows an important diagnostic value and can corroborate the early diagnosis of PROM, being a simple, rapid and accessible test. This study does not intend to establish it as the only diagnostic method, but as a presumptive test to be used in localities where there is no access to the totality of complementary tests to make an accurate diagnosis of PROM. The qualitative β-hCG test, given its practicality, advantage and easy accessibility, is a useful tool for the detection of PROM in any part of the national territory where there is no gynecology and obstetrics service.
REFERENCES
1. Azam A, Husnain H MI. Premature rupture of membranes: Diagnostic accuracy of ß-hCG test in vaginal washings taking amniotic fluid pooling as gold standard of diagnosing PROM. Prof Med J. 2018;25(2):168-72. [ Links ]
2. Ruanphoo P, Phupong V. Evaluation of the performance of the insulin-like growth factor-binding protein-1/alpha-fetoprotein test in diagnosing ruptured fetal membranes in pregnant women. J Perinatol. 2015;35(8):558-60. https://doi.org/10.1038/jp.2015.6 [ Links ]
3. Abdelazim I, Al-Sherbeeny M, Ibrahim M, Fahmy A, Rabei N, Khalifa AA. Insulin-like growth factor binding Protein-1/ alpha-fetoprotein versus placental alpha microglobulin-1 for diagnosis of premature fetal membranes rupture. Acta Medica Int. 2016;3(1):69. DOI: 10.5530/ami.2016.1.15 [ Links ]
4. Lee S, Romero R, Shin J, Chaemsaithong PKJ YB. A transcervical amniotic fluid collector: a new medical device for the assessment of amniotic fluid in patients with ruptured membranes. J Perinat Med. 2015;43(4):381-9. doi: 10.1515/jpm-2014-0276. [ Links ]
5. López-Osma FA, Ordoñez-Sánchez SA. Rotura prematura de membranas fetales: de la fisiopatología hacia los marcadores tempranos de la enfermedad. Rev Colomb Obstet Ginecol. 2006;57(4):279-90. [ Links ]
6. Koch M, Seltzer P, Pezzini A DM. Rotura prematura de membranas. Rev posgrado VIa Cátedra Med. 2008;182. [ Links ]
7. Buziquia C, Shozo N, Nery L, Polato A, Yurie L, Getirana R et al. Qualitative determination of human chorionic gonadotropin in vaginal washings for the early diagnosis of premature rupture of fetal membranes. Rev Bras Ginecol Obs. 2017;39(07):317-21. doi: 10.1055/s-0037-1603939 [ Links ]
8. Urdaneta-García A, Reyna-Villasmil E, Mejía-Montilla J, Torres-Cepeda D, Santos-Bolívar J, Reyna-Villasmil N, et al. Gonadotropina coriónica en flujo vaginal para el diagnóstico de rotura prematura de membranas. Rev Chil Obstet Ginecol. 2014;79(6):502-7. http://dx.doi.org/10.4067/S0717-75262014000600007 [ Links ]
9. Bahasadri S, Kashanian M, Khalili S. Evaluation of vaginal fluid ß-human chorionic gonadotrophin for the diagnosis of preterm premature rupture of membranes. J Obstet Gynaecol Res. 2013;39(4):777-82. DOI: 10.1111/jog.12012 [ Links ]
10. Kim YH, Park YW, Kwon HS, Kwon JY, Kim BJ. Vaginal fluid ß-human chorionic gonadotropin level in the diagnosis of premature rupture of membranes. Acta Obstet Gynecol Scand. 2005;84(8):802-5. [ Links ]
11. Abbas A, El-Shorbagy S, El-Bandary A E-AA. Evaluation of vaginal fluid ß-human chorionic gonadotropin for diagnosis of premature rupture of membranes. Med J Cairo Univ. 2018;86(March):597-603. [ Links ]
12. RCOG Green-top Guideline No. 73. R Coll Obstet Gynaecol. 2019;73:153-66. [ Links ]
13. Rogers LC, Scott L, Block JE. Accurate point-of-care detection of ruptured fetal membranes: improved diagnostic performance characteristics with a monoclonal/polyclonal immunoassay. Clin Med Insights Reprod Heal. 2016;10:CMRH. S38386. doi: 10.4137/CMRH.S38386 [ Links ]
14. Temel O, Çögendez E, Selçuk S, Asoglu MR, Kaya E. ß-human chorionic gonadotropin assay in vaginal washing fluid for the accurate diagnosis of premature rupture of membranes during late pregnancy. J Turkish Ger Gynecol Assoc. 2013;14(4):201-4. doi: 10.5152/jtgga.2013.65624 [ Links ]
15. Remberto L, Bustos A, Aguíñiga GR, Virgen R, Morales P. Sensibilidad y especificidad de la detección cualitativa de b -HCGh en el lavado cervicovaginal para el diagnóstico de rotura prematura de membranas. Arch Inv Mat Inf. 2013;V:41-6. [ Links ]
16. Barooti E, Darvish S, Kariman N, Yazdanpanah G. Comparison of human chorionic gonadotropin test and amnisure test for diagnosis of premature rapture of membrane. Med Lab J. 2019;13(1):28-32. DOI: 10.29252/mlj.13.1.28 [ Links ]
17. Martínez JJR, López JAS, López RA, Benavides JLI. Comparación entre dos pruebas diagnósticas de rotura prematura de membranas. Ginecol Obstet Mex. 2012;80(3):195-200. [ Links ]
18. Méndez-González JA, Aguirre-Ramos G, Álvarez-Valero R, Velázquez-Magaña M, Rojas-Poceros G. Hormona gonadotropina coriónica humana vaginal versus cristalografía y papel de nitrazina para el diagnóstico de rotura prematura de membranas. Anales médicos (México, D.F.). 2007;52(1):22-6. [ Links ]
19. Anai T, Tanaka Y, Hirota Y, Miyakawa I. Vaginal fluid hCG levels for detecting premature rupture of membranes. Obstet Gynecol. 1997;89(2):261-4. [ Links ]
20. Ni CY, Jia WX, Yi WM, Feng LH, Yu LZ. Practicability of using vaginal fluid markers in detecting premature rupture of membranes. Ann Clin Biochem. 2003;40(5):542-5. [ Links ]
21. Ghasemi M, Jaami R, Alleyassin A, Ansarimoghaddam A. The value of urea, creatinine, prolactin, and beta sub-unit of human chorionic gonadotropin of vaginal fluid in the diagnosis of premature preterm rupture of membranes in pregnancy. Turkish J Obstet Gynecol. 2016;13(2):62-6. doi: 10.4274/tjod.48902 [ Links ]
22. Bufalino G, Aponte A, García BF, Fabrega R PC. ß-hCG en fluidos vaginales como marcador de rotura prematura de membranas. Rev Obs Ginecol. 2003;63(4):181-6. [ Links ]
23. Carranza Lira S, Negrete López M, Quinzaños Fresnedo C, Leaños Miranda A. Utilidad de la detección cualitativa de hCG en el lavado cervicovaginal para el diagnóstico de rotura prematura de membranas. Ginecol Obstet Mex. 2009;77(3):142-6. [ Links ]
24. Eldaly A, Omran E, Youssef MA, Abdallah A, Metwally A, Haggag H, et al. Use of beta subunit of human chorionic gonadotropin assay as a diagnostic tool for prelabor rupture of membranes. J Matern Neonatal Med [Internet]. 2019;32(12):1965-70. https://doi.org/10.1080/14767058.2017.1422712 [ Links ]
25. Esim E, Turan C, Unal O, Dansuk R CB. Diagnosis of premature rupture of membranes by identification of beta-HCG in vaginal washing fluid. Eur J Obs Gynecol Reprod Biol. 2003;107(1):37-40. [ Links ]
6The study was submitted to the bioethics committee of the Catholic University of Honduras, informed consent was obtained from each participant and from the Mario Catarino Rivas Hospital, where the study was carried out
Cite as: Zúñiga Girón L, Alas Pineda C, Ratliff Subillaga P, Ponce Barahona F, Bejarano S, Aeschlimann Canizales F, Murillo Guerrero D, Calix Cruz K, Valladares Flores M. Qualitative human beta chorionic gonadotropin for the diagnosis of premature rupture of membranes in Honduras. Rev Peru Gynecol Obstet. 2022;68(1). DOI: 10.31403/rpgo.v68i2402
Received: August 10, 2021; Accepted: October 16, 2021











 texto en
texto en 



