Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.1 Lima ene./mar. 2022 Epub 24-Feb-2022
http://dx.doi.org/10.31403/rpgo.v68i2382
Original article
Placental anastomoses in monochorionic twin pregnancies: study using vascular injection techniques and relationship with fetal complications
1. Fetal Surveillance Unit-Hospital Nacional Edgardo Rebagliati Martins, EsSalud, Lima, Peru
Introduction:
Twin pregnancies are classified into two groups: monochorionic (MC) and dichorionic (DC). MC twins are 5 to 6 times more likely to have an adverse perinatal outcome. The study of a group of 22 placentas from patients with monochorionic twin pregnancy who presented with complications such as feto-fetal transfusion syndrome (FFTS), twin anemia polycythemia sequence (TAPS), twin reverse arterial perfusion syndrome (TRAP) and selective intrauterine growth restriction (sIUGR) is presented.
Objective:
To determine the predominant types of anastomoses in placentas with feto-fetal transfusion syndrome, twin anemia polycythemia sequence, reverse arterial perfusion syndrome and selective intrauterine growth restriction. Methodology: The placental injection technique was applied for the recognition of anastomoses.
Results:
The mean number of anastomoses per placenta in STFF, which was the most severe complication, was 8.2 ± 2.2. The AV and VA anastomoses predominated in 83%. There were signs of placental discordance in 30% of placentas, and 40% of placentas presented velamentous cord insertion.
Conclusions:
Vascular anastomoses are not only involved in the etiology of the main pathologies of monochorionic gestations, but also influence their management. We believe that an adequate placental study of each of these cases by means of the placental vascular injection technique would be essential in centers that aspire to develop differentiated fetal management for each of these complications.
Key words: Twin pregnancy; monochorionic; Placental circulation; Chorion; Placental anastomosis
Introducción
Twin pregnancies are classified into two groups: monochorionic (MC) and dichorionic (DC). MC twins are 5 to 6 times more at risk of having an adverse perinatal outcome1. Adverse perinatal outcomes are due to unbalanced vascular anastomoses connecting the circulation of both twins. Although these anastomoses can also occur in dichorionic placentas, they are extremely rare2.
These anastomoses are responsible for a blood exchange between the two fetuses, which, according to their complexity and type, will lead to the appearance of complications such as feto-fetal transfusion syndrome (FFTS), twin anemia polycythemia sequence (TAPS), reverse arterial perfusion syndrome (TRAP) and selective intrauterine growth restriction (sIUGR)3.
In the present work we studied a group of placentas from patients with monochorionic gestational pregnancy who presented complications such as FFTS, TAPS, TRAP and selective IUGR with the aim of evaluating the predominant types of placental anastomoses.
Our center has initiated a fetal surgery program, specifically in placental laser surgery. In this context, it is important to examine the placentas of monochorionic gestations and apply the injection technique in order to detect the different anastomoses, understand the implications of their distribution and how this will determine the spectrum of different complications. It is also necessary to study placentas after placental laser surgery and to evaluate the new characteristics of these anastomoses as an important indicator of the outcome of the surgery.
Methods
Placentas from monochorionic pregnancies that terminated at the Edgardo Rebagliati Martins National Hospital, EsSalud, Lima, Peru, were evaluated during 2 years. Twenty-two placentas from monochorionic pregnancies with and without complications were studied. The diagnosis of chorionicity was typed with first trimester ultrasound. The placental injection technique was used to recognize the anastomoses3,4.
To perform the injection technique, after birth, clamps were used for each of the cords and the placenta was preserved in sterile water and not in formalin, since this would make it impossible to apply the injection technique. A detailed inspection of the placental fetal surface was performed, describing the type of cord insertion (central, marginal, eccentric or velamentous) and the number of vessels in the cord. The placentas were studied in the first 48 to 72 hours after delivery, because later the technique presents unsatisfactory results due to the collapse of the vessels. Before starting the vascular injection, the placenta was washed with saline solution and the placental fetal side was freed from the amniotic membrane to facilitate exploration and visualization of the anastomosis.
The cords were cut 6 cm from the placental insertion and then the umbilical vessels were cannulated with two catheters: one 5F for the umbilical vein and another 3F for the umbilical artery. Once the vessels were cannulated, we started the application of liquid tempera diluted in a 20 mL syringe loaded with different colors. The technique of displacement of the injected material was applied in order to spread the dye to the most distant vessels, since these will be the ones that will finally anastomose with the vessels of the other placental territory. Once the placental vessels were cannulated and colored, the vascular equator was examined and the number, caliber and type of anastomoses observed were determined (Figure 1). The vascular anastomoses were classified into 3 types: arterio-arterial (AA), veno-venous (VV) and arterio-venous (AV) or veno-arterial (VA) anastomoses3.
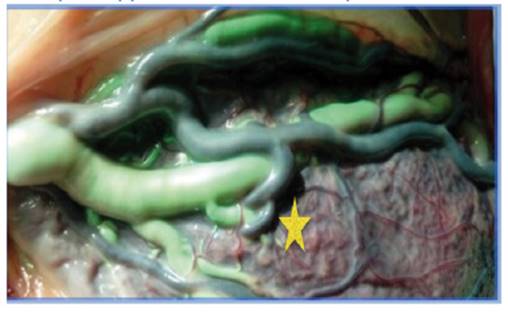
Figure 1 Unidirectional AV anastomosis in placenta without complications (star) (Author's own elaboration).
The first two types are superficial, with bidirectional blood flow and directly join the arteries and veins of two umbilical cords; while AV and VA anastomoses are formed at a deep capillary level within the shared cotyledons and only allow unidirectional blood flow. After application of the injected color dye, we evaluated whether the color blended and crossed the vascular equator, which occurs in AA and VV anastomoses; the opposite occurs with the color dye in AV or VA anastomoses, as they do not mix and do not cross the vascular equator.
The monochorionic twin pregnancies evaluated were classified into 2 groups: without and with complications. In the second group, feto-fetal transfusion syndrome (FFTS), twin anemia polycythemia sequence (TAPS), twin reverse arterial perfusion (TRAP) sequence and selective intrauterine growth restriction of one of the fetuses (sIUGR) were considered.
A pregnancy complicated with feto-fetal transfusion syndrome was considered when oligohydramnios (deepest vertical pocket ≤2 cm) present in the sac of one twin with a collapsed bladder (the donor) and polyhydramnios (deepest vertical pocket ≥8 cm) present in the sac of the other twin with a distended bladder (the recipient) were detected on ultrasound5.
A pregnancy with TAPS was categorized as a complicated pregnancy when discordant middle cerebral artery peak systolic velocity (MCA-PSV) measurements were found on sonographic follow-up: increased middle cerebral artery peak systolic velocity (MCA-PSV>1.5 MoM) in the donor twin (indicative of fetal anemia) and a decrease (MCA-PSV <1.0 MoM) in the recipient twin (indicative of polycythemia)6.
A complicated monochorionic pregnancy with selective IUGR was considered when growth restriction was detected in only one fetus. The fetus with the lowest weight had to have a fetal weight estimated by ultrasound to be below the 10th percentile7,8.
Concomitantly, the fetus with selective IUGR was classified according to the umbilical artery flow pattern, according to the Gratacos classification8. This classification considers 3 types, depending on the umbilical artery flow pattern:
Type I: if the twin with IUGR presents normal flow pattern.
Type II: persistently absent or reversed diastolic flow.
Type III: intermittent diastolic flow (normal, absent and reversed in the same exam).
The twin reversed arterial perfusion (TRAP) sequence was defined by the presence of a malformed fetus that has a rudimentary or absent heart, and there is no direct placental perfusion. But it is perfused through an arterio-arterial communication from the structurally normal co-twin (or pump fetus) and then doubly deoxygenated; after perfusing the acardium, it returns to the pump fetus through a veno-venous connection9.
Results
Twenty-two placentas from monochorionic twin gestations were evaluated. Vascular anastomosis (AA, AV, VA and/or VV) were identified in 100% of the placentas studied.
Four of the placentas evaluated were monochorionic with no complications. Vascular anastomoses are always present in monochorionic placentas10 and their distribution and type will determine the evolution of the pregnancy11. The mean number of anastomoses per placenta for this first group was 7.2 ± 3.2. The prevalence of AV and VA anastomoses predominated in 75% (3/4) followed by AA anastomoses in 50% (2/4) and VV anastomoses in 25% (1/4) of the cases. There were no signs of placenta-placental discordance or velamentous insertion cord.
FFTS is the most severe complication in monochorionic pregnancies and develops in approximately 10% of MC gestations12. In our series, we injected a total of 6 placentas with non-laser-treated FFTS (Figure 2). The mean number of anastomoses per placenta for this second group was 8.2 ± 2.2. AV and VA anastomoses predominated in 83% (5/6), followed by VV anastomoses in 66% (4/6) and AA anastomoses in 33% (2/6). There were signs of placental discordance in 30% of placentas and 40% of placentas had velamentous cord insertion. One of the cases that presented FFTS was referred to undergo placental laser. The procedure was performed at 26 weeks and at 3 weeks there was an increase of systolic peak in the middle cerebral artery of one of the fetuses, which was classified as TAPS and a severe polyhydramnios-oligohydramnios sequence at 30 weeks, and the patient underwent cesarean section due to acute deterioration of one of the twins. Placental revision detected the coagulation line with a very small residual anastomosis (Figure 3). One of the twins died one week after birth; the other twin remained in the intensive care unit for 3 weeks due to prematurity and associated complications. At the time of the present study, he was under ambulatory controls according to protocol.
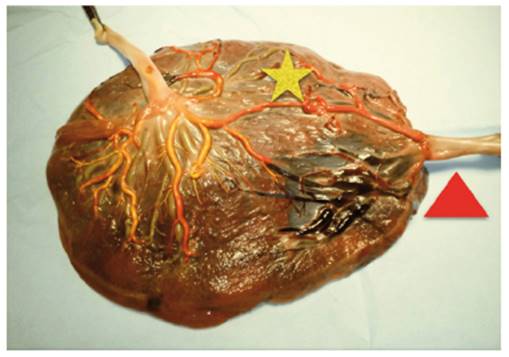
Figure 2 Placenta complicated with FFTS. It presents an arterio-arterial anastomosis (star) and a velamentous insertion (triangle): 5 arteriovenous anastomoses (A-V) come from the donor fetus to the recipient fetus (Author's own elaboration).
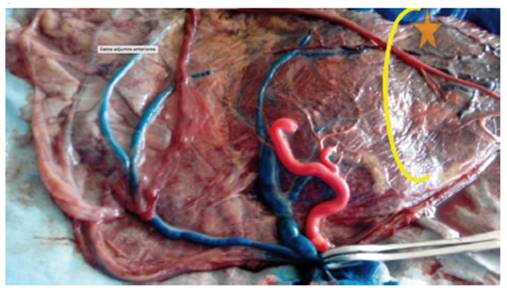
Figure 3 Placenta of a complicated pregnancy with post laser FFTS: the coagulation line with a residual anastomosis (star) is evident (Author's own elaboration).
During the study period there were 3 cases of pregnancies complicated with the twin anemia-polycythemia sequence (TAPS): two of them spontaneous and one post-laser. TAPS can occur spontaneously or after laser surgery for FFTS due to small residual anastomoses13. For this third group, the mean number of anastomoses per placenta was 3.2 ± 2.2. AV and VA anastomoses predominated with 66% (2/3) and AA anastomoses were present in 33% (1/3). There was no evidence of veno-venous anastomoses (Figure 4). There were signs of placental discordance in 66% of placentas, and 33% of placentas with velamentous insertion cord.
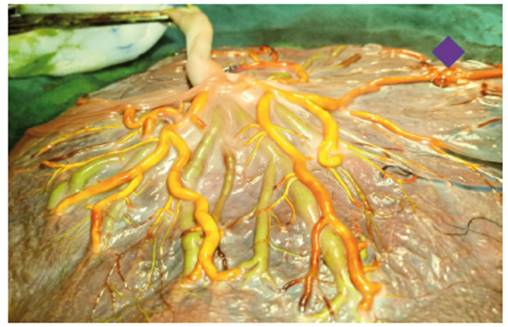
Figure 4 Placenta of a twin pregnancy complicated with TAPS. Anastomosis A-A (rhombus). Monochorionic twins, one fetus weighing 2,200 g and the other 1,800 g. The diagnosis was made at 31 weeks. Caesarean section was scheduled at 32 weeks and a transfusion was necessary at birth (Author's own elaboration).
Selective intrauterine growth restriction (sIUGR) affects approximately 10 to 20% of monochorionic pregnancies14. There were 8 cases in this fourth group: four cases as type 1 sIUGR, two cases as type 2 and two cases as type 3 of the Gratacos classification8. The mean number of anastomoses per placenta was 10.2 ± 5.6. The prevalence of AA anastomoses, AV-VA anastomoses and VV anastomoses was 100% (8/8), 87% (7/8) and 37% (3/8), respectively. sIUGR are strongly associated with unequal placental territory: a large placental distribution for the large twin and a small placental distribution for the twin with restricted growth (Figure 5). It was present in all 8 cases, 7 of which had velamentous insertion of the cord. The results of placental discordance and type of cord insertion in placentas with sIUGR as well as the types of anastomoses in our cohort are shown in Table 1.
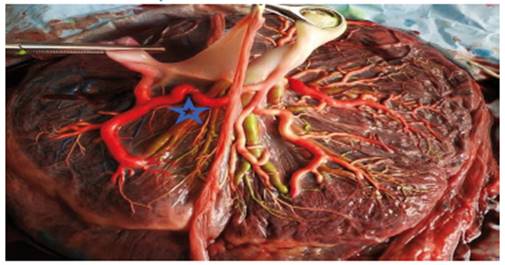
Figure 5 Placenta of complicated pregnancy with selective IUGR. Selective IUGR pattern type 3. Adjacent cords with a large A-A (star), 1 V-V, 5 A-V and 5 V-A anastomoses (Author's own elaboration).
Table 1 Placental characteristics of the monochorionic gestations.
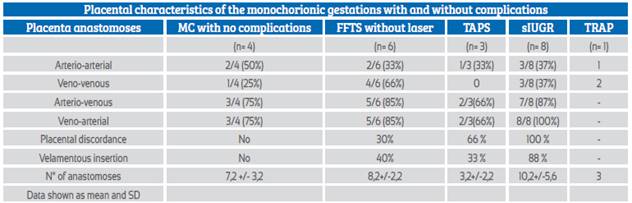
MC=monochorionic, STFF= feto-fetal transfusion syndrome, TAPS= twin anemia polycythemia sequence, sIUGR= selective intrauterine growth restriction, TRAP=twin reversed arterial perfusion, SD= standard deviation
Twin reversed arterial perfusion (TRAP) complicates approximately 1 in 35,000 pregnancies 15,16. The pathogenesis of TRAP is related to the presence of a large AA anastomosis. One case of TRAP sequence was evaluated in the study period. One arterio-arterial (AA) and two veno-venous (VV) anastomoses were found, with no velamentous insertion.
Discussion
In our study, the presence of placental anastomoses in monochorionic gestation was present in 100% of cases, which is in agreement with other authors who describe them in over 95% in different studies of placental angioarchitecture17,18. In the literature it is reported that monochorionic placentas frequently present vascular anastomoses, especially arterio-arterial (A-A), with a high frequency of AV and VA anastomoses19; the latter were the most frequent in our study. VV anastomoses are generally less frequent: 25% according to some series20. However, most CM pregnancies develop well without complications, suggesting a balanced blood exchange between twins.
In the present work, four monochorionic placentas were injected and did not present associated pathology (Figure 1). The mean number of vascular anastomoses in normal MC placentas varies in different studies between 2 and 7.9, which is in agreement with our findings19,21. This may be attributed to different techniques of placental examination: from naked eye detection to injection of colored dye. Anastomoses in this group were mostly unidirectional, reaching 75% and, on the other hand, AA anastomoses reached 50%. The finding of a higher number of unidirectional anastomoses compared to bidirectional anastomoses reflects the concept that the balance between blood flows depends not only on the number of anastomoses, but also on other variables, such as the caliber of the anastomoses, which may influence the balance in blood exchange between twins and may be reflected in not developing complications. The high rate of AA anastomoses is typical of uncomplicated CM and it is believed that such AA anastomoses prevent the development of various complications, due to compensation through bidirectional blood flow. The role of VV anastomoses is unclear and remains to be elucidated 22.
FFTS is the most serious complication in MC twin pregnancies and develops in approximately 10% of MC pregnancies4. The mean number of anastomoses in FFTS is similar to that in uncomplicated MC placentas. However, in FFTS placentas, the net blood flow and/or the number of AV anastomoses are not balanced, resulting in donor-to-recipient transfusion. Postnatal injection studies such as in vivo fetoscopic observations indicate the presence of at least one unidirectional arteriovenous (A-V) anastomosis as an anatomic prerequisite for the development of FFTS11,23. Although there is no single pattern of anastomoses, the presence of an arterio-arterial anastomosis appears to be protective16. As such, an arterio-arterial anastomosis was present in only 2 of 7 placentas with FFTS; the other five had no AA anastomoses. FFTS is the most important cause of death and disability in a monochorionic twin pregnancy24,25.
Currently, laser coagulation of all anastomoses at the level of the placental equator is the best alternative to treat this syndrome26. The aim of the laser application is to cure the disease by disconnecting the 2 fetal circulations. Successful interruption leads to normalization of urine output, amniotic fluid volumes and cardiac function in the recipient twin.
After laser treatment, there is a 50-60% survival of both twins and an 80% survival of at least one twin, making this treatment the treatment of choice today27. One of the cases in our series underwent placental laser treatment for a FFTS. It has been described that after placental surgery, the type and size of the omitted anastomoses correlate with the subsequent outcome27. As such, omitted large arteriovenous anastomoses may lead to recurrent FFTS or double demise, unless a compensatory arterio-arterial anastomosis is also omitted27. On the other hand, omitted small arteriovenous anastomoses may lead to TAPS28. TAPS is associated with small caliber anastomoses, on average 1 mm, that could not be photocoagulated29.
In our series, the MC pregnancy that underwent fetoscopy for FFTS developed an anemia-polycythemia sequence syndrome (TAPS) 3 weeks post-procedure, leading to termination of the pregnancy due to fetal deterioration. The postpartum placental study showed small residual anastomoses of less than 1 mm (Figure 3).
Therefore, we recommend placental injection of all placentas that underwent laser treatment, except those with single intrauterine death and delayed delivery, since anastomoses can no longer be documented. Placental injection is also a means of quality control and can improve surgical technique30.
The reverse arterial perfusion sequence (TRAP) complicates approximately 1 in 35,000 pregnancies31. In the TRAP sequence, blood flows from an umbilical artery of the pump twin in the reverse direction into the umbilical artery of the perfused (acardiac) twin through an arterio-arterial anastomosis and usually returns through a veno-venous anastomosis to the pump twin31. TRAP is an infrequent condition and complicates approximately 1% of monochorionic twin pregnancies. The condition is associated with a high risk of perinatal death caused by a combination of high output heart failure and premature delivery related to polyhydramnios32. In our hospital, 2 cases have been described in 7 years; the last one was treated during the study period, so the placenta could be checked by the injection technique. The findings determined a large AA anastomosis and the return to the pump twin through a large VV anastomosis (Figure 6). The high morbidity of the healthy twin can be improved by an intervention to stop the circulation of the acardiac twin. The type of technique will depend on the clinical presentation and may consist of coagulation of the umbilical cord of the acardiac twin33,34.
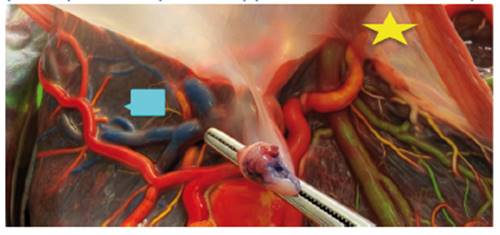
Figure 6 Placenta of TRAP twins. It presents 1 A-A (star) and 1 V-V anastomosis (rectangle) (Author's own elaboration).
Selective intrauterine growth restriction (sIUGR) affects approximately 10 to 20% of MC twin pregnancies compared to 8% in dichrorionic twins22,35. sIUGR produces a high mortality and morbidity rate among MC twins3,10. Placentas with sIUGR are characterized by the presence of a large discordance in the placental territory and higher rates of insertion of the velamentous cord14. Unequal placental exchange appears to be the most important determinant of discordant growth in monochorionic twins36. The number of anastomoses in the selective IUGR fetuses evaluated in our series was similar to those in normal MC placentas. However, almost all sIUGR placentas had an AA anastomosis with a significantly larger diameter compared with normal MC placentas. sRCIU have been described to be strongly associated with unequal placental exchange. In our study, the degree of territorial discordance in fetuses with selective IUGR was 80%.
An important fact was the high frequency of velamentous cord insertion in IUGR placentae. In our series it was 88%, significantly higher compared to normal MC placentas. The velamentous cord insertion belonged to the growth-restricted fetus in 90% of the cases. Therefore, in many cases it may be an indicator of poor perinatal prognosis. For this reason, we consider it important to pinpoint the site of cord insertion in the scanning of twins, which can be observed by sonographers with experience in the second trimester ultrasound examination, and cases of early detection have been described as early as the first trimester37.
Although it is true that we have had few cases in the study period, given the prevalence of these complications, the series represents a significant number of twin pregnancies with complications in our institution.
Vascular anastomoses are determinant in defining the main complications of monochorionic pregnancies. Their nature and the possibility of surgical approach influence their management. Therefore, we believe that an adequate placental study in each monochorionic placenta by means of the placental injection technique is indispensable in centers that aspire to develop differentiated fetal therapy management for each of these complications.
Finally, placental examination provides a good learning experience for any fetal medicine specialist seeking to embark on intrauterine endoscopic laser surgery.
REFERENCES
1. Acosta-Rojas R, Becker J, Munoz-Abellana B, Ruiz C, Carreras E, Gratacos E; Catalunya and Balears Monochorionic Network. Twin chorionicity and the risk of adverse perinatal outcome. Int J Gynaecol Obstet. 2007;96:98-102. https://doi.org/10.1016/j.ijgo.2006.11.002 [ Links ]
2. Lewi L, Gucciardo L, Van Mieghem T. Monochorionic diamniotic twin pregnancies: natural history and risk stratification. Fetal Diagn Ther. 2010;27:121-33. https://doi.org/10.1159/000313300 [ Links ]
3. Lewi L, Cannie M, Blickstein I. Placental sharing, birthweight discordance and vascular anastomoses in monochorionic diamniotic twin placentas. Am J Obstet Gynecol. 2007;197:587.e1-8. https://doi.org/10.1016/j.ajog.2007.05.009 [ Links ]
4. Lewi L, Deprest J, Hecher K. The vascular anastomoses in monochorionic twin pregnancies and their clinical consequences. AJOG. 2013;208(1):19-30.https://doi.org/10.1016/j.ajog.2012.09.025 [ Links ]
5. Quintero RA. Twin-twin transfusion syndrome. Clin Perinatol. 2003;30:591-600. https://doi.org/10.1016/s0095-5108(03)00051-4 [ Links ]
6. Slaghekke F, Kist WJ, Oepkes D, Pasman SA, Middeldorp JM, Klumper FJ, et al. Twin anemia-polycythemia sequence: diagnostic criteria, classification, perinatal management and outcome. Fetal Diagn Ther. 2010;27:181-90. https://doi.org/10.1159/000304512 [ Links ]
7. DePaepe ME, Shapiro S, Young L, Luks FI.Placental characteristics of selective birth weight discordance in diamniotic- monochorionic twin gestations. Placenta. 2010;31:380-6. https://doi.org/10.1016/j.placenta.2010.02.018 [ Links ]
8. Gratacós E, Lewi L, Munoz B. A classification system for selective intrauterine growth restriction in monochorionic pregnancies according to umbilical artery Doppler flow in the smaller twin. Ultrasound Obstet Gynecol. 2007;30:28-34. https://doi.org/10.1002/uog.4046 [ Links ]
9. Van Allen MI, Smith DW, Shephard TH. Twin reversed arterial perfusion (TRAP) se- quence: a study of 14 twin pregnancies with acardius. Semin Perinatol. 1983;7:285-93. PMID: 6658475(Indexed for MEDLINE) [ Links ]
10. Lewi L, Gucciardo L, Huber A, Jani J, Van Mieghem T, Done E, et al. Clinical outcome and placental characteristics of monochorionic diamniotic twin pairs with early- and late-onset discordant growth. Am J Obstet Gynecol. 2008;199:511e1-7. https://doi.org/10.1016/j.ajog.2008.03.050 [ Links ]
11. Diehl W, Hecher K, Zikulnig L, Vetter M, Hackelöer BJ. Placental vascular anastomoses visualized during fetoscopic laser surgery in severe mid-trimester twin-twin transfusion syndrome. Placenta. 2001;22:876-81. https://doi.org/10.1053/plac.2001.0710 [ Links ]
12. Lewi L, Jani J, Blickstein I, Huber A, Gucciardo L, Van Mieghem T, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynec. 2008;199: 514e1- 8. https://doi.org/10.1016/j.ajog2008.03.050 [ Links ]
13. Robyr R, Lewi L, Salomon LJ, Yamamoto M, Bernard JP, Deprest J, et al. Prevalence and management of late fetal complications following successful selective laser coagulation of chorionic plate anastomoses in twin-to-twin transfusion syndrome. Am J Obstet Gynecol. 2006;194:796-803. https://doi.org/10.1016/j.ajog.2005.08.069 [ Links ]
14. Lopriore E, Pasman SA, Klumper FJ, Middeldorp JM, Walther FJ, Oepkes D, et al. Placental characteristics in growth-discordant monochorionic twins: a matched case-control study. Placenta. 2012;33:171-4. https://doi.org/10.1016/j.placenta.2011.12.004 [ Links ]
15. Diehl W, Hecher K. Selective cord coagulation in acardiac twins. Semin Fetal Neonatal Med. 2007;12:458-63. doi.10.1016.siny.2007.06.008 [ Links ]
16. Moore TR, Gale S, Benirschke K. Perinatal outcome of forty-nine pregnancies complicated by acardiac twinning. Am J Obstet Gynecol. 1990;163:907-12. https://doi.org/10.1016/0002-9378(90)91094-s [ Links ]
17. Lanna MM, Consonni D, Faiola S, Schena V, Ratti M, Ferrazzi E, Rustico MA. Color-dye injection of monochorionic placentas and correlation with pregnancy complications. Placenta. 2015Oct;36(10):1095-9. https://doi.org/10.1016/j.placenta.2015.07.129 [ Links ]
18. Lipa M, Kosinski P, Stanirowski P, Wielgos M, Bomba-Opon D. Vascular anastomoses in intrauterine growth in monochorionic twins. J Perinat Med. 2020Jul 28;48(6):539-43. https://doi.org/10.1515/jpm-2020-0028 [ Links ]
19. Atallah A, Bolze PA, Buenerd A, Marino S, Massardier J, Gaucherand P, Massoud M. Injection des anastomoses vasculaires pour la compréhension des complications propres aux grossesses monochoriales [Macroscopic description of placental vascular anastomoses after dye injection for the comprehension of monochorionic pregnancy complications]. Gynecol Obstet Fertil Senol. 2017May;45(5):269-75. French. https://doi.org/10.1016/j.gofs.2017.03.002 [ Links ]
20. Denbow ML, Cox P, Taylor M, Hammal DM, Fisk NM. Placental angioarchitecture in monochorionic twin pregnancies: relationship to fetal growth, maternofetal transfusion syndrome, and pregnancy outcome. Am J Obstet Gynecol 2000;182:417-26. https://doi.org/10.1016/s0002-9378(00)70233-x [ Links ]
21. Bajoria R. Vascular anatomy of monochorionic placenta in relation to discordant growth and amniotic fluid volume. Hum Reprod. 1998;13:2933-40. https://doi.org/10.1093/humrep/13.10.2933 [ Links ]
22. Umur A, van Gemert MJ, Nikkels PG, Ross MG. Monochorionic twins and twin-twin transfusion syndrome: the protective role of arterio-arterial anastomoses. Placenta. 2002;23:201-9. https://doi.org/10.1053/plac.2001.0758 [ Links ]
23. Bermúdez C, Becerra CH, Bornick PW, Allen MH, Arroyo J, Quintero RA. Placental types and twin-twin transfusion syndrome. Am J Obstet Gynecol. 2002;187:489-94. https://doi.org/10.1067/mob.2002.124280 [ Links ]
24. Mahieu-Caputo D, Meulemans A, Martinovic J. Paradoxic activation of the renin-angiotensin system in twin-twin transfusion syndrome: an explanation for cardiovascular disturbances in the recipient. Pediatr Res. 2005;58:685-8. https://doi.org/10.1203/01.PDR.0000180558.03164.E8 [ Links ]
25. Ortibus E, Lopriore E, Deprest J. The pregnancy and longterm neurodevelopmental outcome of monochorionic diamniotic twin gestations: a multicenter prospective cohort study from the first trimester onward. Am J Obstet Gynecol. 2009;200:494.e1-8. https://doi.org/10.1016/j.ajog.2009.01.048 [ Links ]
26. Roberts D, Gates S, Kilby M, Neilson JP. Interventions for twin-twin transfusion syn- drome: a Cochrane review. Ultrasound Obstet Gynecol. 2008;31:701-11. https://doi.org/10.1002/uog.5328 [ Links ]
27. van Gemert MJ, Umur A, Tijssen JG, Ross MG. Twin-twin transfusion syndrome: etiology, severity and rational management. Curr Opin Obstet Gynecol. 2001;13:193-206. https://doi.org10.1097/00001703-200104000-00015 [ Links ]
28. Lewi L, Jani J, Cannie M. Intertwin anastomoses in monochorionic placentas after fetoscopic laser coagulation for twinto- twin transfusion syndrome: is there more than meets the eye? Am J Obstet Gynecol. 2006;194:790-5. https://doi.org/10.1016/j.ajog.2005.08.062 [ Links ]
29. Lopriore E, Slaghekke F, Middeldorp JM, Klumper FJ, Oepkes D, Vandenbussche FP, et al. Residual anastomoses in twin-to-twin transfusion syndrome treated with selective fetoscopic laser surgery: localization, size, and consequences. Am J Obstet Gynecol. 2009;201:66.e1-4. https://doi.org/10.1016/j.ajog.2009.01.010 [ Links ]
30. Lopriore E, Deprest J, Slaghekke F. Placental characteristics in monochorionic twins with and without twin anemia-polycythemia sequence. Obstet Gynecol. 2008;112:753-8. https://doi.org/10.1097/AOG.0b013e318187e1ff [ Links ]
31. Diehl W, Hecher K. Selective cord coagulation in acardiac twins. Semin Fetal Neonatal Med. 2007;12:458-63. https://doi.org/10.1016%2Fj.siny.2007.06.008 [ Links ]
32. Moore TR, Gale S, Benirschke K. Perinatal outcome of forty-nine pregnancies complicated by acardiac twinning. Am J Obstet Gynecol. 1990;163:907-12. https://doi.org/10.1016/0002-9378(90)91094-s [ Links ]
33. Hecher K, Lewi L, Gratacos E, Huber A, Ville Y, Deprest J, et al. Twin reversed arterial perfusion: fetoscopic laser coagulation of placental anastomoses or the umbilical cord. Ultrasound Obstet Gynecol. 2006;28:688-91. https://doi.org/10.1002/uog.3816 [ Links ]
34. Lee H, Wagner AJ, Sy E, Ball R, Feldstein VA, Goldstein RB, Farmer D. Efficacy of radiofrequency ablation for twin-reversed arterial perfusion sequence. Am J Obstet Gynecol. 2007; 196:4591.e1-4 https://doi.org/10.1016/j.ajog.2006.11.039 [ Links ]
35. De Paepe ME, Shapiro S, Young L, Luks FI. Placental characteristics of selective birth weight discordance in diamniotic- monochorionic twin gestations. Placenta. 2010;31:380-6. https://doi.org/10.1016/j.placenta.2010.02.018 [ Links ]
36. Fick AL, Feldstein VA, Norton ME, Wassel Fyr C, Caughey AB, Machin GA. Unequal placental sharing and birth weight discordance in monochorionic diamniotic twins. Am J Obstet Gynecol. 2006;195:178-83. https://doi.org/10.1016/j.ajog.2006.01.015 [ Links ]
37. Sepulveda W, Rojas I, Robert JA, Schnapp C, Alcalde JL. Prenatal detection of velamentous insertion of the umbilical cord: a prospective color Doppler ultrasound study. Ultrasound Obstet Gynecol. 2003;21:564-9. https://doi.org/10.1002/uog.132 [ Links ]
Received: May 24, 2021; Accepted: December 21, 2021; pub: February 22, 2022











 texto en
texto en 



