Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Peruana de Ginecología y Obstetricia
On-line version ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.1 Lima Jan./Mar. 2022 Epub Feb 24, 2022
http://dx.doi.org/10.31403/rpgo.v68i2403
Brief originals
Case series of right aortic arch: prenatal diagnostic approach
1. Hospital Nacional Daniel Alcides Carrión. Callao, Perú
2. Clinica Santa Isabel, Lima, Peru
3. Centro de Medicina Fetal: CENMEF, Lima, Perú
The right aortic arch is a product of abnormal involution of the embryonic vascular arches. In recent years, fetal diagnosis has become more frequent with the use of routine ultrasonography of the heart and great vessels. The finding of a right aortic arch involves many aspects that may affect the prognosis of the fetus; therefore, the exhaustive study must be systematized. Below, we present a series of six cases of prenatal diagnosis and suggest an evaluation algorithm.
Key words: Right aortic arch; Vascular ring; Echocardiography; fetal
Introduction
The normal formation of the aortic arch occurs during the embryonic period from the involution of the pharyngeal vascular arches in a complex process. The third arch forms the carotid arteries, the fourth arch the aortic arch itself and the sixth the ductus arteriosus with the pulmonary arteries. The laterality of the aortic arch is caused by the involution of the right arch. Alterations of the aortic arch involve not only the laterality of the arch, but also the persistence of both arches, the interruption of the arch and abnormalities in the birth of the cephalic vessels1. Right aortic arch (RAA) has an incidence of approximately 0.1% of gestations. Fetal diagnosis in recent years has become more frequent with the use of the ultrasonographic approach to the heart and great vessels recommended by the International Society of Ultrasonography in Obstetrics and Gynecology (ISUOG), which assesses crosslinking of the great vessels in the superior mediastinum2,3.
The finding of an RAA involves many aspects that may affect the prognosis of gestation; therefore, the exhaustive examination should be systematized. Below, we present a series of prenatal diagnostic cases and suggest an evaluation algorithm.
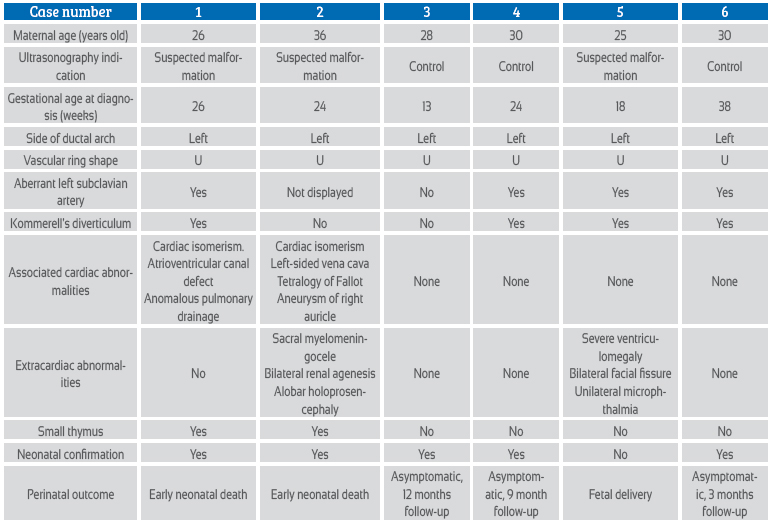
Case series report
The series of cases occurred over a period of 4 years. The main patient data are abstracted in Table 1. For the diagnosis, fetal echocardiography with Doppler was performed in all cases, for evidence of the shape of the vascular ring of the right aortic arch and to search for aberrant left subclavian artery (ALSA) or Kommerell's diverticulum (Figures 1 to 5). In the most recent cases, STIC (spatio-temporal image correlation) technology was used for three-dimensional reconstruction of the aortic arch, Kommerell's diverticulum and aberrant left subclavian artery (Figures 3, 4 and 5).
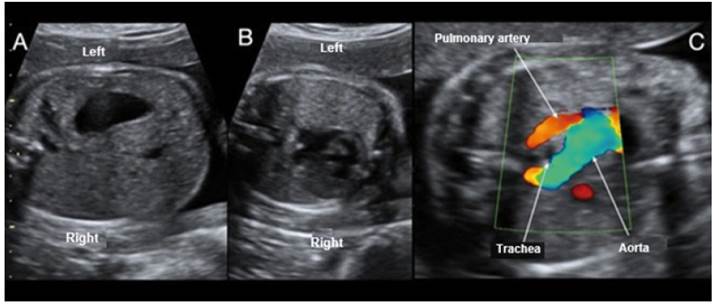
Figure 1 Case 1. A: View of visceral situs with stomach on the left side; the inferior vena cava is not visualized. B: Axial section at the level of 4 cardiac chambers, with silhouette on the right side. C: Doppler section of 3 tracheal vessels, showing the aorta passing through the right side of the trachea.
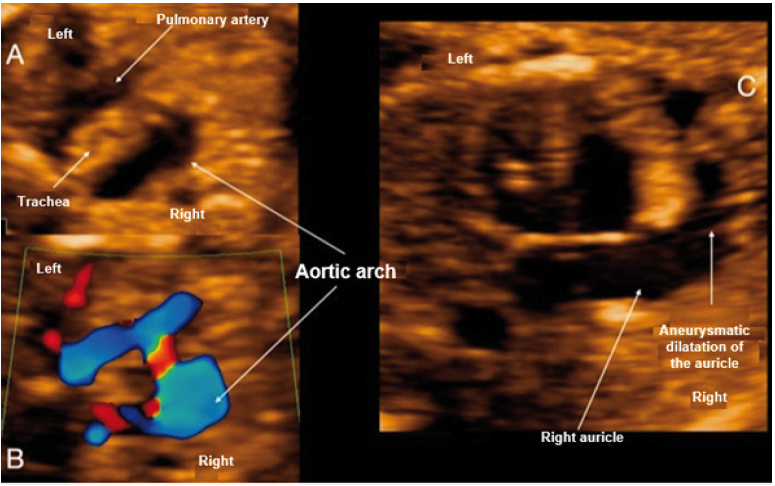
Figure 2 Case 2. A: Section of 3 vessels trachea showing the aorta passing through the right of the trachea. B: Doppler section of 3 vessels trachea forming a U-shape with the ductus arteriosus and the trachea in the center. C: View of 4 cardiac chambers, showing marked dilatation of the right atrial appendage.
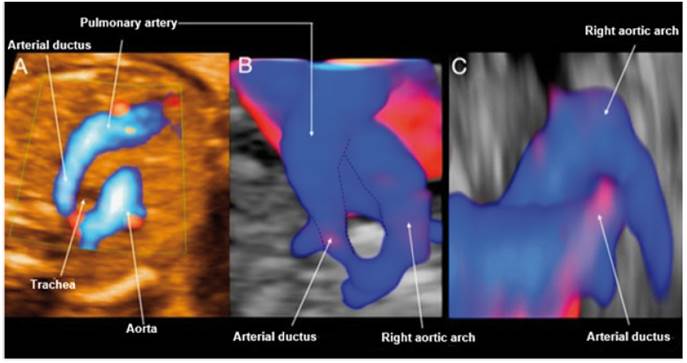
Figure 3 Case 3. A: Doppler section of the 3-vessel trachea forming a U-shape with the ductus arteriosus and the trachea in the center. B: STIC reconstruction of the 3-vessel trachea with Doppler. C: Reconstruction with STIC in sagittal view from the left, showing the entrance of the ductus arteriosus in the right aortic arch without visible Kommerell's diverticulum.
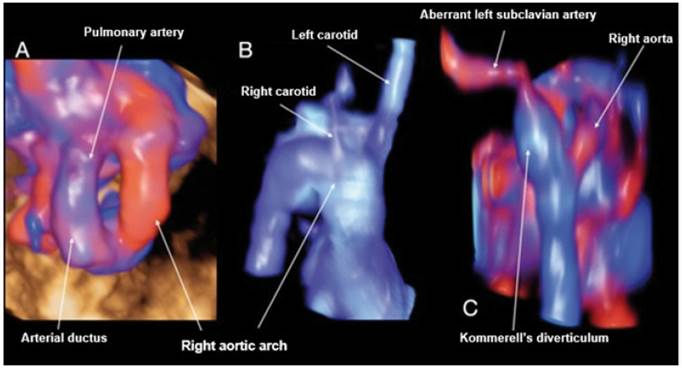
Figure 4 Case 4. A: STIC reconstruction of the 3 vessels trachea vessels with Doppler, showing the U-shape. B: STIC reconstruction in frontal view of the right aortic arch, showing a first vessel corresponding to the left carotid followed by the right carotid. C: Reconstruction with STIC in posterior view of the aortic arch and descending aorta, showing the entrance of the ductus arteriosus with the Kommerell's diverticulum visible, from which the ALSA arises.
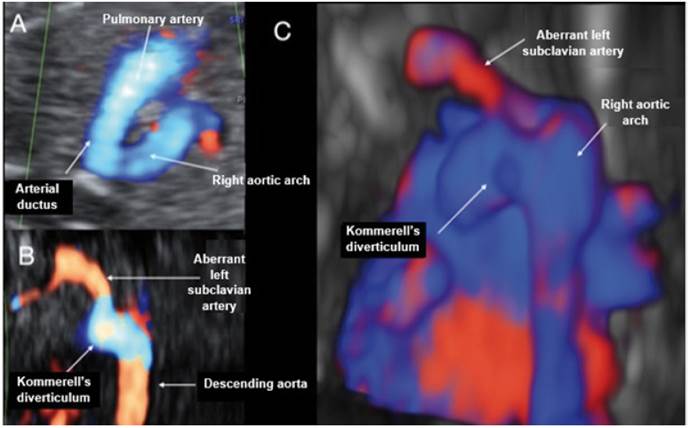
Figure 5 Case 5. A: Doppler section of the 3-vessel trachea forming a U-shape with the ductus arteriosus and the trachea in the center. B: Coronal view at the level of the descending aorta, where the dilatation of Kommerell's diverticulum can be seen at the entry of the ductus into the aorta and how ALSA is born. C: Reconstruction with STIC in posterior view of the aortic arch and descending aorta, showing the entry of the ductus arteriosus with the Kommerell's diverticulum visible, from where ALSA arises.
Discussion
RAA is diagnosed by prenatal ultrasound in the three-vessel and tracheal section of the heart examination, the normal finding being that the aorta and ductus arteriosus are on the left side of the trachea. On the other hand, the right aortic arch will be on the right side of the trachea, closer to the superior vena cava2. It is necessary to determine the side on which the ductus arteriosus runs, since if it is on the right side, the possibility of a vascular ring is minimal, but there is a greater association with other cardiopathies. On the other hand, if it is on the left side (normal), there is a greater risk of forming a vascular ring (around the trachea and esophagus) and the characteristic U-shaped conformation of the vessels will be seen (Figures 1C, 2A, 2B, 3C, 5A). In our series, we found the ductal arch on the left side in all cases studied, associating in all of them the characteristic U-shape of the vascular ring1. Likewise, it is necessary to extend the evaluation with the visualization of the origin of the aortic vessels (the use of Doppler is absolutely necessary), focusing on the search in ALSA (artery that arises from the descending part of the aorta crossing behind the esophagus) and in the presence of Kommerell's diverticulum (remnant of the involuted embryonic left aortic arch, where the ductus arteriosus usually drains in cases of RAA or the aberrant left subclavian artery has its origin)1 (Figures 4C, 5B and 5C). Of the 6 cases presented in this series, in 4 of them it was possible to visualize ALSA associated with Kommerell's diverticulum at its origin.
There are up to 5 varieties in the birth of the vessels of an RAA, but two are the most important in the fetus1. The most frequent form is characterized by aberrant left subclavian artery (ALSA) arising from Kommerell's diverticulum, with little association with added cardiac malformations, but which can form symptomatic vascular ring (Figures 4B, 4C, 5B and C). The second form in frequency is the variant with mirror aortic vessels, with a high association to cardiac malformations and little risk of forming a vascular ring1,4. The evaluation of both varieties requires great experience of the examiner. Three-dimensional vascular reconstruction could also be used for a more detailed analysis, by means of spatio-temporal image correlation (STIC). We recommend reviewing the algorithm published by Wang for further evaluation5. Vigneswaran6, in an extensive series of cases of isolated RAA (138), reports 70 fetuses with a variant with mirror aortic vessels and 5 with ALSA. In contrast, the data published by Campanale7 (37 fetuses) show 56% of ALSA and only 10% of mirror aortic vessels.
The cardiac malformations frequently associated with RAA are conotruncal malformations, including tetralogy of Fallot (especially the variant with pulmonary valve agenesis); others are septal defects and isomerisms4,8-11. The series of 98 cases published by Miranda9 graphs these data, finding tetralogy of Fallot in 50% of cases, septal defects in 11% and 8.5% of isomerism. Likewise, these findings are consistent with the 50 cases reported by Zidere7. In our series, 2 of the cases showed associated cardiac anomalies, in both cases with isomerism (Table 1).
The prevalence of extracardiac malformations associated with RAA is variable; it is reported between 14.6% and 31%9,12. It is necessary to point out that the association with extracardiac malformations increases the risk of 22q11.2 deletion11. The extracardiac features that should be looked for when 22q11.2 is suspected are facial dysmorphisms, renal malformations and thymus hypoplasia13. For prenatal evaluation of thymus hypoplasia we recommend the Chaoui technique (thymus/thorax ratio)14. In accordance with this prevalence, in two cases (40%) extracardiac anomalies were evidenced, with the first case presenting bilateral renal agenesis, sacral myelomeningocele and allobar holoprosencephaly; while in the second case bilateral facial clefting, unilateral microphthalmia and severe ventriculomegaly were found. In addition, thymus hypoplasia was found in two cases, but genetic testing was not performed (Table 1).
The association of RAA with chromosomopathies is 9% overall; however, if only cases without heart disease or extracardiac malformation are considered, it decreases to 4.6%12. The study of chromosome 22q11.2 deletion is recommended if RAA is associated with other cardiopathies (especially conotruncal: tetralogy of Fallot, truncus arteriosus, coarctation of the aorta), when finding an absent/hypoplastic thymus and/or extracardiac malformations (facial, soft palate, renal)15. Thus, Zidere, in a series of 75 cases with high association to cardiopathies, found a 24% prevalence of 22q11.2 deletion8. The case is different when finding an 'isolated' RAA, where the probability is 4%-8.5%, as shown in multiple series and meta-analyses4,6,12,16.
The long-term prognosis in cases without other malformations is determined by the symptomatology of the vascular ring, which is formed by the right aortic arch, the left aberrant left subclavian artery, and the left descending aorta with the left ductus arteriosus, which finally joins the left subclavian artery with the left pulmonary artery. Although most children affected by vascular rings are asymptomatic, the incidence of respiratory or esophageal symptoms in the first years of life in children with prenatal diagnosis of RAA varies between 13% and 25%4,6,12,17. Of the 6 cases reported in our series, one of them ended in fetal death, two had an early neonatal death, and the other three were followed up postnatally between 3 and 12 months of life, being asymptomatic until the date of this publication (Table 1).
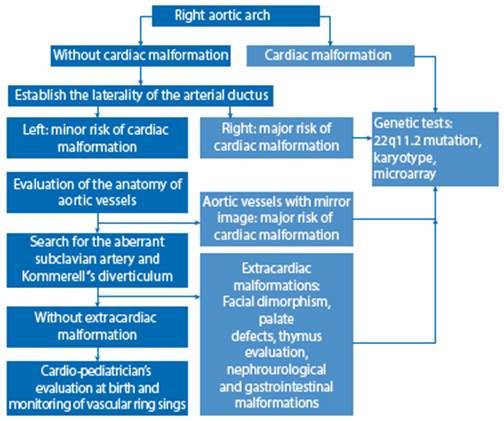
In conclusion, when a right aortic arch is found, it is necessary to establish a detailed study and sequential study, in order to determine the pertinence of the genetic diagnostic tests, as we propose in the evaluation algorithm (Figure 6); this will allow us to provide adequate counseling to the mother on prognosis and follow-up at birth.
REFERENCES
1. Hanneman K, Newman B, Chan F. Congenital variants and anomalies of the aortic arch. Radiographics. 2017;37(1):32- 51. doi:10.1148/rg.2017160033 [ Links ]
2. Yagel S, Arbel R, Anteby EY, Raveh D, Achiron R. The three vessels and trachea view (3VT) in fetal cardiac scanning. Ultrasound Obstet Gynecol. 2002;20(4):340-5. doi:10.1046/j.1469-0705.2002.00801.x [ Links ]
3. International Society of Ultrasound in Obstetrics and Gynecology, Carvalho JS, Allan LD, Chaoui R,Copel JA, DeVorre GR,et al. ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013;41(3):348-59. doi:10.1002/uog.12403 [ Links ]
4. Razon Y, Berant M, Fogelman R, Amir G, Birk E. Prenatal diagnosis and outcome of right aortic arch without significant intracardiac anomaly. J Am Soc Echocardiogr. 2014;27:1352- 8. doi: 10.1016/j.echo.2014.08.003 [ Links ]
5. Wang Y, Fan M, Siddiqui FA, Wang M, Sun W, Sun X, et al. Strategies for accurate diagnosis of fetal aortic arch anomalies: benefits of three-dimensional sonography with spatiotemporal image correlation and a novel algorithm for volume analysis. J Am Soc Echocardiogr. 2018;31(11):1238- 51. doi: 10.1016/j.echo.2018.07.010 [ Links ]
6. Vigneswaran TV, Allan L, Charakida M, Durward A, Simpson JM, Nicolaides KH, Zidere V. Prenatal diagnosis and clinical implications of an apparently isolated right aortic arch. Prenat Diagn. 2018;38(13):1055-61. doi: 10.1002/pd.5388. PMID: 30421794. [ Links ]
7. Campanale CM, Pasquini L, Santangelo TP, Iorio FS, Bagolan P, Sanders SP, Toscano A. Prenatal echocardiographic assessment of right aortic arch. Ultrasound Obstet Gynecol. 2019;54(1):96-102. doi: 10.1002/uog.20098 [ Links ]
8. Zidere V, Tsapakis EG, Huggon IC, Allan LD. Right aortic arch in the fetus. Ultrasound Obstet Gynecol. 2006;28(7):876-81. doi:10.1002/uog.3841 [ Links ]
9. Miranda JO, Callaghan N, Miller O, Simpson J, Sharland G. Right aortic arch diagnosed antenatally: associations and outcome in 98 fetuses. Heart. 2014;100(1):54-9. doi:10.1136/heartjnl-2013-304860 [ Links ]
10. Peng R, Xie HN, Zheng J, Zhou Y, Lin MF. Fetal right aortic arch: associated anomalies, genetic anomalies with chromosomal microarray analysis, and postnatal outcome. Prenat Diagn. 2017;37(4):329-35. doi:10.1002/pd.5015 [ Links ]
11. Berg C, Bender F, Soukup M, Geipel A, Axt-fliedner R, Breuer J, et al. Right aortic arch detected in fetal life. Ultrasound Obstet Gynecol. 2006;28(7):882-9. doi:10.1002/uog.3883 [ Links ]
12. D'Antonio F, Khalil A, Zidere V, Carvalho JS. Fetuses with right aortic arch: a multicenter cohort study and meta-analysis. Ultrasound Obstet Gynecol. 2016;47(4):423-32. doi:10.1002/uog.15805 [ Links ]
13. Besseau-Ayasse J, Violle-Poirsier C, Bazin A, Gruchy N, Moncla A, Girard F. et al. A French collaborative survey of 272 fetuses with 22q11.2 deletion: ultrasound findings, fetal autopsies and pregnancy outcomes. Prenat Diagn. 2014;34(5):424-30. doi:10.1002/pd.4321 [ Links ]
14. Chaoui R, Heling KS, Lopez AS, Thiel G, Karl K. The thymic- thoracic ratio in fetal heart defects: a simple way to identify fetuses at high risk for microdeletion 22q11. Ultrasound Obstet Gynecol. 2011;37(4):397-403. doi:10.1002/uog.8952 [ Links ]
15. Goldmuntz E. 22q11.2 deletion syndrome and congenital heart disease. Am J Med Genet C Semin Med Genet. 2020;184(1):64 72. doi:10.1002/ajmg.c.31774 [ Links ]
16. Perolo A, De Robertis V, Cataneo I, Volpe N, Campobasso G, Frusca T, et al. Risk of 22q11.2 deletion in fetuses with right aortic arch and without intracardiac anomalies. Ultrasound Obstet Gynecol. 2016;48(2):200-3. doi: 10.1002/uog.15766 [ Links ]
17. Mogra R, Kesby G, Sholler G, Hyett J. Identification and management of fetal isolated right-sided aortic arch in an unselected population. Ultrasound Obstet Gynecol. 2016;48(6):739-43. doi:10.1002/uog.15892 [ Links ]
Received: June 16, 2021; Accepted: September 20, 2021; pub: March 29, 2022











 text in
text in