Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.2 Lima abr./jun. 2022 Epub 06-Jul-2022
http://dx.doi.org/10.31403/rpgo.v68i2409
Original paper
Red cell distribution width in the second trimester of pregnancy as a predictor of preeclampsia
1. Assistant physician of the Obstetrics and Gynecology Service. Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela
2. Faculty of Medicine, University of Zulia, Venezuela
Objective:
To establish the usefulness of red cell distribution width in the second trimester of pregnancy as a predictor of the development of preeclampsia.
Design:
Case-control study. Institution: Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela. Participants: Pregnant women between 17 and 20 weeks who attended prenatal consultation and were followed up until to term.
Methods:
Blood samples were taken and followed up until delivery to establish if they developed preeclampsia.
Main outcome measures:
General characteristics, values of red cell distribution width and prognostic efficacy.
Results:
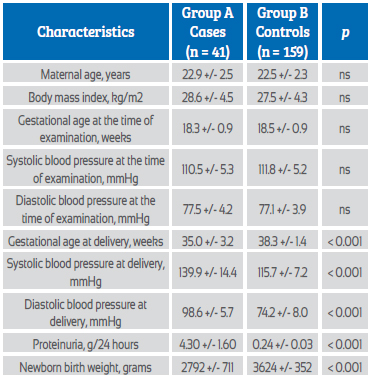
Cases were 41 pregnant women who developed preeclampsia (group A) and 463 pregnant women were considered as controls (group B). No statistically significant differences were found in maternal age, gestational age, and systolic and diastolic blood pressure at the time of ultrasound (p = ns). Gestational age at the time of diagnosis of preeclampsia in group A was 35.0 +/- 3.2 weeks. Significant differences were found in red cell distribution width values between patients in group A (14.5 +/- 2.3%) and patients in group B (13.8 +/- 1.8%; p = 0.039). A cut-off value of 14% presented a value under the curve of 0.576 with sensitivity of 63.4%, specificity of 49.7%, positive predictive value of 10.0% and negative predictive value of 93.9%.
Conclusion:
The values of red cell distribution width values in the second trimester are not useful in the prediction of preeclampsia.
Key words: Erythrocyte indices; Red cell distribution width; Preeclampsia; prediction; Pregnancy
Introduction
Preeclampsia is characterized by hypertension and proteinuria and is one of the main causes of maternal and perinatal morbidity and mortality1. The underlying pathology for its occurrence is still not fully understood. It has been suggested that the main pathophysiological mechanism is altered placentation, producing inadequate invasion by the cytotrophoblast and generalized maternal endothelial dysfunction. The clinical manifestations are associated with the latter, which causes vasoconstriction and end-organ injury2. Increased erythropoietic activity secondary to underlying placental hypoxia has also been described3.
Red cell distribution width (RCDW, an analysis that measures the variation in the volume and size of red blood cells or erythrocytes) is a useful hematological parameter for evaluating the heterogeneity of red blood cell size, both due to destruction and production deficits that lead to an increase in erythrocyte volume4. It is used as an aid in the differential diagnosis of hypochromic anemias. But recently several investigations have shown its association with increased mortality in patients with cardiovascular disease5,6, as well as a marker of systemic inflammatory response in other chronic diseases7. The type and kind of association are unclear, although it has been suggested that inflammation and nutritional deficiencies, especially in iron metabolism, lead to increased RCDW values. This bone marrow dysfunction secondary to systemic inflammation results in increased ineffective erythropoiesis which contributes to increased anisocytosis, reflected by RCDW values8.
Although the relationship between RCDW and hypertension has been clearly demonstrated, there is controversial data on its association with the diagnosis of preeclampsia and the evidence is still scarce on its usefulness in the prediction of the syndrome3,9-11. The aim of this study was to establish the usefulness of red cell distribution width in the second trimester of pregnancy as a predictor of the development of preeclampsia.
Methods
A prospective, explanatory study was conducted in nulliparous pregnant women with singleton pregnancies who were attended at the outpatient prenatal clinic of the Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela, between January 2014 and September 2021. The study protocol was approved by the Hospital Ethics Committee before the start of the research and written consent was obtained from all patients.
Once the patients were selected for the study, a data collection form was filled out that included: patient identification, personal and gynecological-obstetrical history, prenatal control, gestational age (by date of last menstrual period or first trimester ultrasound) and leukocyte count values. Gestational age was calculated on the date of the last menstrual period and corrected by ultrasound if measurements during the first trimester showed a difference of more than 7 days. All pregnancies were followed until delivery and were categorized according to the development of preeclampsia (cases; group A) or not (controls; group B).
Patients with a diagnosis of preeclampsia were included. This was considered when systolic blood pressure was equal to or greater than 140 mmHg and/or diastolic blood pressure equal to or greater than 90 mmHg, confirmed by 6 h or more difference. Proteinuria was defined as 300 mg or more of protein in a 24-h sample after 20 weeks of gestation. Blood pressure was measured in a sitting position after 15 min rest, using a standard mercury sphygmomanometer with a 14-cm cuff. Systolic and diastolic blood pressure (taken relative to the fifth Korotkoff noise) was located relative to the nearest 2 mmHg point. The palpatory method was used to verify auscultatory readings of systolic blood pressure. Systolic and diastolic blood pressures were calculated from the average blood pressure of each arm.
Pregnant women with a diagnosis of polyhydramnios, third trimester hemorrhage (placental abruption, placenta previa), suspected intrauterine growth restriction of the fetus (head circumference, abdominal circumference and femur length less than the 10th percentile of reference with postnatal confirmation of weight less than the 10th percentile of reference), severe preeclampsia with multiorgan manifestations, fetal heart rate alterations, multiple gestations, presence of intrauterine or active maternal infection, chronic hypertensive disease (hypertension before 20 weeks of pregnancy), cardiac, hematologic, hepatic, renal or chronic systemic disease, pre- or gestational diabetes mellitus, smoking, those pregnant women in whom blood samples could not be obtained. Patients who refused to participate in the research were also excluded.
Antecubital vein blood samples were collected at the time of routine ultrasound evaluation in all selected pregnant women during the second trimester of pregnancy (17-20 weeks). Two milliliters of blood were taken and ethylenediaminetetraacetic acid was added and immediately analyzed to obtain the RCDW value, using the same automatic quantitative hematology analyzer. This describes the percentage variation (being statistically a coefficient of variation) of red blood cell size, where its formula is: (standard deviation/mean corpuscular volumen) × 1004.
The values obtained were presented as mean +/- standard deviation. The Kolmogorov-Smirnov test was used to test the normal distribution of the data (p > 0.05). Student's t test for unrelated samples was used for cluster analysis and to compare continuous variables. The accuracy of RCDW values for predicting the development of preeclampsia is presented in terms of sensitivity, specificity, positive predictive value, and negative predictive value. Operator-receptor analysis was used to determine the best cutoff value. This was established by the highest sensitivity and specificity represented by the highest Youden index. p < 0.05 was considered statistically significant.
Results
The results were obtained from the measurements of 504 primigravid pregnant women, of whom 41 patients (8.1%) developed preeclampsia (group A) and 463 pregnant women (91.9%) were considered controls (group B). The general characteristics of the 2 groups of pregnant women are shown in Table 1. No statistically significant differences were found in maternal age, gestational age, and systolic and diastolic blood pressure at the time of ultrasound (p = ns). Gestational age at diagnosis of preeclampsia in group A was 35.0 +/- 3.2 weeks. Statistically significant differences were observed in gestational age at delivery, systolic and diastolic blood pressure at delivery, 24-hour proteinuria, and newborn birth weight between the 2 groups of pregnant women (p < 0.0001).
Figure 1 shows the mean RCDW values. Statistically significant differences were observed in the mean values between patients in group A (14.5 +/- 2.3%) compared to patients in group B (13.8 +/- 1.8%; p = 0.039).
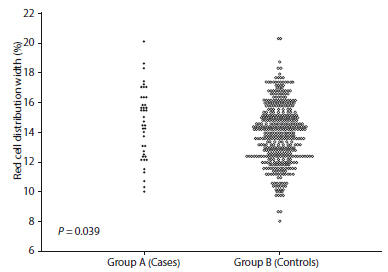
Figure 1 Red cell distribution width values in the second trimester in pregnant women who subsequently developed preeclampsia compared to healthy pregnant controls.
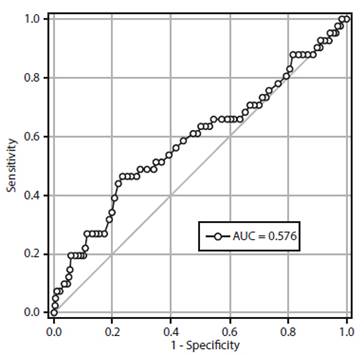
Figure 2 shows the operator-receptor curve for the accuracy of RCDW values in predicting preeclampsia. A cutoff value of 14% mg/dL presented a value under the curve of 0.576 (95% confidence interval (95% CI), 0.473-0.679) and sensitivity values of 63.4% (95% CI; 48.1-76.4), specificity of 49.7% (95% CI; 45.1-54.2), positive predictive value of 10.0% (95% CI; 6.9-14.3) and negative predictive value of 93.9% (95% CI; 90.1-96.4), together with a positive likelihood ratio of 1.26 (95% CI; 0.98-1.62) and positive likelihood ratio of 0.74 (95% CI; 0.48-1.12). The diagnostic accuracy was 50.8% (95% CI, 46.4-55.1).
Discussion
Elevated RCDW values reflect heterogeneity of erythrocyte size, which is caused by disturbance in erythrocyte degradation or maturation. It is a widely available hematologic index showing variation in erythrocyte volume called anisocytosis, which is commonly used to discriminate and differentiate between types of anemia12. It may also reflect a state of chronic inflammation, which may be associated with increased risk of cardiovascular disease5. Research results demonstrate that RCDW values are not useful in the prognosis of preeclampsia, even though preeclamptic women have significantly higher values compared to healthy normotensive pregnant women.
Although the etiology of preeclampsia is unknown, altered placentation and immune tolerance, in addition to the accentuated maternal vascular inflammatory response, are thought to be responsible for its occurrence13. Fibrinoid material and foam cells located around the spiral arteries cause decreased blood flow, leading to hypoxia14. Increased stimulation of erythropoietin production secondary to underlying placental hypoxia has been described in the preeclamptic arteries3. The increased inflammatory response in preeclampsia increases the destruction of erythrocytes by interacting with oxygen radicals and proteolytic enzymes. Following this destruction, increased concentrations of some catabolic products, such as erythrocyte membrane protein 3, have been reported15,16.
The findings of this research are supported by previous studies that have shown increased RCDW values in preeclamptic compared to healthy normotensive pregnant women9-11. Viana-Rojas et al9 found significantly increased values in preeclamptic women. They also showed that severe preeclamptic women had higher values compared to mild preeclamptic women. On the other hand, RCDW values are significantly increased in severe preeclamptic women compared to healthy pregnant controls and mild preeclamptic women11. Yucel et al.10 found that severe preeclamptic women had significantly higher values compared with controls, but the test was not useful in differentiating severe from mild preeclamptic women. However, there is previous research that found no association between RCDW values and the occurrence of the syndrome3.
Sen-yu et al.17 conducted a study similar to this one, in which they analyzed RCDW values in the second trimester and, like the results of this study, found higher values in those pregnant women who developed preeclampsia in the third trimester. However, in contrast to the results reported in this investigation, their response operator curve analysis showed that the determination had clinical value for the prediction of the onset and development of preeclampsia, which was contrary to what was found in this group of patients.
There are studies that show an association between increased RCDW values and biomarkers of oxidative stress, inflammation, malnutrition, and renal dysfunction8. Of these, oxidative stress and inflammation have been studied in somewhat greater depth as determinants of variation, so that these processes may affect the presence of anisocytosis. Regarding the inflammatory state, there are conflicting results, as some studies have found a statistical relationship between RCDW values and various systemic inflammatory parameters7,8, while others have found no significant associations18. Although the mechanism linking it to hypertension is not clearly understood, inflammation is the most likely explanation for this phenomenon19. It has also recently been observed that RCDW values are associated with the presence of hypertension and is an indicator of poor prognosis in acute myocardial infarction and heart failure20,21. It has been proposed that inflammation may increase values, due to reticuloendothelial blockade, causing alterations in iron metabolism for erythropoiesis and in the response to erythropoietin. This could suppress erythrocyte maturation, shortening their half-life and leading to immature erythrocytes entering the circulation22,23.
The increase in RCDW values in pregnant women who subsequently develop preeclampsia can be explained by several possible mechanisms, the most likely being an increase in the systemic inflammatory response20. This fact is supported by the positive correlation between C-reactive protein and RCDW values in preeclampsia11. Previous studies have shown that preeclampsia is associated with increased concentrations of tumor necrosis factor-alpha and interleukin-624,25. In addition, inflammatory cytokines cause immature erythrocytes to enter the circulation by affecting the maturation process 11.
This research has some limitations. The study was conducted in a single hospital, which makes it difficult to extrapolate the results. However, the sample of pregnant women included were obtained consecutively among those who attended the hospital, and their characteristics do not indicate significant bias samples. Tests for hemoglobin electrophoresis, serum vitamin B12 and folate concentrations, serum iron concentrations, total iron binding capacity and transferrin saturation, which are important to exclude hemoglobinopathies, early macrocytosis due to folic acid or cobalamin deficiency, which may increase RCDW values, were not performed. On the other hand, differences in hematological equipment would make comparisons between different countries and populations difficult, because each laboratory defines a normal range and, in addition, each equipment is calibrated differently.
Several studies have proposed that RCDW could be a useful parameter for the diagnosis or prognosis of different diseases20,26. But it is still unclear whether anisocytosis (reflected by RCDW values) could be the cause and/or consequence of the underlying disease. Nevertheless, RCDW is an easy, inexpensive, routinely reported parameter that could provide significant diagnostic and prognostic information in subjects diagnosed with hypertension and preeclampsia.
REFERENCES
1. Bajpai D. Preeclampsia for the nephrologist: Current understanding in diagnosis, management, and long-term outcomes. Adv Chronic Kidney Dis. 2020;27(6):540-50. doi: 10.1053/j.ackd.2020.05.001 [ Links ]
2. Opichka MA, Rappelt MW, Gutterman DD, Grobe JL, McIntosh JJ. Vascular dysfunction in preeclampsia. Cells. 2021;10(11):3055. doi: 10.3390/cells10113055 [ Links ]
3. Abdullahi H, Osman A, Rayis DA, Gasim GI, Imam AM, Adam I. Red blood cell distribution width is not correlated with preeclampsia among pregnant Sudanese women. Diagn Pathol. 2014;9:29. doi: 10.1186/1746-1596-9-29 [ Links ]
4. Xanthopoulos A, Tryposkiadis K, Dimos A, Bourazana A, Zagouras A, Iakovis N, et al. Red blood cell distribution width in elderly hospitalized patients with cardiovascular disease. World J Cardiol. 2021;13(9):503-13. doi: 10.4330/wjc.v13.i9.503 [ Links ]
5. Pieszko K, Hiczkiewicz J, Budzianowski P, Budzianowski J, Rzezniczak J, Pieszko K, et al. Predicting long-term mortality after acute coronary syndrome using machine learning techniques and hematological markers. Dis Markers. 2019;2019:9056402. doi: 10.1155/2019/9056402 [ Links ]
6. Talarico M, Manicardi M, Vitolo M, Malavasi VL, Valenti AC, Sgreccia D, et al. Red cell distribution width and patient outcome in cardiovascular disease: a ''real-world'' analysis. J Cardiovasc Dev Dis. 2021;8(10):120. doi: 10.3390/jcdd8100120 [ Links ]
7. Yan L, Hu ZD. Red blood cell distribution width, neutrophil- to-lymphocyte ratio, and in-hospital mortality in dyspneic patients admitted to the emergency department. Dis Markers. 2020;2020:8839506. doi: 10.1155/2020/8839506 [ Links ]
8. Lim CJ, Shen Y, Lee SY, Ryu PD. Potential erythropoiesis in the primo-vascular system in heart failure. Adv Exp Med Biol. 2017;977:409-15. doi: 10.1007/978-3-319-55231-6_53 [ Links ]
9. Viana-Rojas JA, Rosas-Cabral A, Prieto-Macías J, Terrones- Saldívar MC, Arcos-Noguez P, Bermúdez-Gómez J, et al. Relation of red cell distribution width and mean platelet volume with the severity of preeclampsia. Rev Med Inst Mex Seguro Soc. 2017;55(2):176-81. [ Links ]
10. Yücel B, Ustun B. Neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, mean platelet volume, red cell distribution width and plateletcrit in preeclampsia. Pregnancy Hypertens. 2017;7:29-32. doi: 10.1016/j.preghy.2016.12.002 [ Links ]
11. Kurt RK, Aras Z, Silfeler DB, Kunt C, Islimye M, Kosar O. Relationship of red cell distribution width with the presence and severity of preeclampsia. Clin Appl Thromb Hemost. 2015;21(2):128-31. doi: 10.1177/1076029613490827 [ Links ]
12. Thompson CA, Litin SC, Bundrick JB. Clinical pearls in hematology, 2016. Dis Mon. 2016;62(7):214-22. doi: 10.1016/j.disamonth.2016.05.003 [ Links ]
13. Guerby P, Tasta O, Swiader A, Pont F, Bujold E, Parant O, et al. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021;40:101861. doi: 10.1016/j.redox.2021.101861 [ Links ]
14. Forstner D, Guettler J, Gauster M. Changes in maternal platelet physiology during gestation and their interaction with trophoblasts. Int J Mol Sci. 2021;22(19):10732. doi: 10.3390/ijms221910732 [ Links ]
15. Wadhwani NS, Sundrani DP, Wagh GN, Mehendale SS, Tipnis MM, Joshi PC, et al. The REVAMP study: research exploring various aspects and mechanisms in preeclampsia: study protocol. BMC Pregnancy Childbirth. 2019;19(1):308. doi: 10.1186/s12884-019-2450-0 [ Links ]
16. Santos-Silva A, Castro EM, Teixeira NA, Guerra FC, Quintanilha A. Erythrocyte membrane band 3 profile imposed by cellular aging, by activated neutrophils and by neutrophilic elastase. Clin Chim Acta. 1998;275(2):185-96. doi: 10.1016/s0009-8981(98)00082-5 [ Links ]
17. Adam I, Mutabingwa TK, Malik EM. Red cell distribution width and preeclampsia: a systematic review and meta-analysis. Clin Hypertens. 2019;25:15. doi: 10.1186/s40885-019-0119-7 [ Links ]
18. Peng Y, Guan X, Wang J, Ma J. Red cell distribution width is correlated with all-cause mortality of patients in the coronary care unit. J Int Med Res. 2020;48(7):300060520941317. doi: 10.1177/0300060520941317 [ Links ]
19. Zheng LH, Liu SY, Hu F, Hu ZC, Shen LS, Wu LM, et al. Relationship between red blood cell distribution width levels and atrial fibrillation in hypertensive patients. J Geriatr Cardiol. 2020;17(8):486-94. doi: 10.11909/j.issn.1671-5411.2020.08.006 [ Links ]
20. Liu J, Yang J, Xu S, Zhu Y, Xu S, Wei L, Qian P, Lv Y, Zhang C, Xing X, Deng Y. Prognostic impact of red blood cell distribution width in pulmonary hypertension patients: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99(16):e19089. doi: 10.1097/MD.0000000000019089 [ Links ]
21. Bao D, Luo G, Kan F, Wang X, Luo J, Jiang C. Prognostic value of red cell distribution width in patients undergoing percutaneous coronary intervention: a meta-analysis. BMJ Open. 2020;10(9):e033378. doi: 10.1136/bmjopen-2019-033378 [ Links ]
22. Hong J, Zhu B, Cai X, Liu S, Liu S, Zhu Q, Aierken X, Aihemaiti A, Wu T, Li N. Assessment of the association between red blood cell distribution width and disease activity in patients with systemic vasculitis. Exp Ther Med. 2021;22(1):691. doi: 10.3892/etm.2021.10123 [ Links ]
23. Hardang IM, Lilleholt K, Hagve TA. Anemia of chronic disease. Tidsskr Nor Laegeforen. 2017;137(17). doi: 10.4045/ tidsskr.16.1128 [ Links ]
24. Reyna E, Mejia J, Reyna N, Torres D, Santos J, Perozo J. Concentraciones de interleucina-6 en preeclámpticas y embarazadas normotensas. Clin Invest Gin Obstet. 2012;39(4):159-163. doi: 10.1016/j.gine.2009.12.010 [ Links ]
25. Reyna-Villasmil E, Mejia-Montilla J, Reyna-Villasmil N, Torres- Cepeda D, Santos-Bolívar J, Perozo-Romero J. Concentraciones del factor de necrosis tumoral alfa en preeclámpticas y embarazadas normotensas sanas. Clin Invest Gin Obstet. 2012;39(6): 236-40. doi: 10.1016/j.gine.2009.12.014 [ Links ]
26. Zhang J, Cao J, Nie W, Shen H, Hui X. Red cell distribution width is an independent risk factor of patients with renal function damage in type 1 diabetes mellitus of children in China. Ann Clin Lab Sci. 2018;48(2):236-41. [ Links ]
Statement of ethical issues
Ethical responsibilities: Protection of persons. The authors declare that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
10Confidentiality of data: The authors declare that they have followed the protocols of the Central Hospital "Dr. Urquinaona" and the University of Zulia on the publication of patient data.
Right to privacy and informed consent: The authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is held by the corresponding author.
Funding: The authors certify that they have not received financial support, equipment, in working personnel or in kind from individuals, public and/or private institutions for the conduct of the study.
Received: December 10, 2021; Accepted: March 19, 2022











 texto en
texto en