Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Peruana de Ginecología y Obstetricia
versión On-line ISSN 2304-5132
Rev. peru. ginecol. obstet. vol.68 no.2 Lima abr./jun. 2022 Epub 06-Jul-2022
http://dx.doi.org/10.31403/rpgo.v68i2410
Original paper
Leukocyte count and neutrophillymphocyte ratio in the second trimester of pregnancy as a predictor of preeclampsia
1Assistant physician of the Obstetrics and Gynecology Service. Hospital Central "Dr. Urquinaona", Maracaibo, Venezuela.
2Faculty of Medicine, University of Zulia, Venezuela
Objective
: To establish the usefulness of leukocyte count and neutrophil/lymphocyte ratio in the second trimester of pregnancy as a predictor of the development of preeclampsia.
Participants
: Pregnant women between 17 and 20 weeks who attended prenatal consultation and were followed up to term. Methods: Blood samples were taken from the pregnant women who were followed until delivery to establish whether they developed preeclampsia. Main outcome measures: General characteristics of the patients, values of leukocytes, neutrophils, lymphocytes, neutrophil/lymphocyte ratio and prognostic efficacy.
Results
: Of the 504 patients selected, 41 pregnant women developed preeclampsia (group A) and 463 pregnant women were considered as controls (group B). No statistically significant differences were found in overall characteristics at baseline (p = ns). The gestational age at the time of diagnosis of preeclampsia in group A was 35.0 +/3.2 weeks. Patients in group A were found to have significantly higher values of leukocytes, neutrophils and neutrophil/lymphocyte ratio along with lower values of lymphocytes compared to patients in group B (p < 0.05). Only the absolute values of neutrophils (area under the curve 0.810) and neutrophil/lymphocyte ratio (area under the curve 0.963) had useful prognostic values for discriminating between patient groups for the development of preeclampsia (p < 0.05). Conclusion: The neutrophil/lymphocyte ratio and absolute neutrophil count in the second trimester of pregnancy are useful tools in the prediction of preeclampsia.
Key words: Leukocytes; Neutrophils; Neutrophil/lymphocyte ratio; Preeclampsia; Prediction.
INTRODUCTION
Preeclampsia is a disorder that affects 4% to 6% of all pregnancies1. Although the mechanisms responsible for its etiology have not been clearly defined, inflammation, endothelial dysfunction, altered angiogenesis, inappropriate placentation, oxidative stress, immunologic and genetic factors are essential components for the development of the syndrome2.
Although preeclampsia is unique to pregnancy in humans and shares pathophysiological characteristics and risk factors (hypertension, diabetes, dyslipidemia and obesity) with cardiovascular disorders in adults, endothelial dysfunction and inflammation are important mechanisms for the onset and development of both conditions3. In addition, immune changes are central to the onset and development of the hypertensive syndrome of pregnancy. It has been suggested that excessive activation and exaggerated immune response by neutrophils and lymphocytes result in the release of inflammatory cytokines and autoantibodies leading to inflammation and endothelial dysfunction(4).
In addition to the probable individual effects of neutrophils and lymphocytes in preeclampsia, the neutrophil/lymphocyte ratio (NLR) has been proposed as a new indicator of increased systemic inflammation. Its predictive and prognostic value has been demonstrated in several cardiovascular diseases, such as hypertension, severity of coronary heart disease, long-term mortality in patients undergoing primary percutaneous coronary intervention, and cardiac mortality in patients with stable coronary artery disease5,6.
NLR has been used to establish the diagnosis and predict the severity of preeclampsia, but so far there are controversial and contradictory results about its role in predicting the syndrome7. The aim of the research was to establish the usefulness of the leukocyte count and neutrophil/ lymphocyte ratio in the second trimester of pregnancy as a predictor of the development of preeclampsia.
METHODS
A prospective, explanatory study was conducted in nulliparous pregnant women with singleton pregnancies who were attended at the outpatient prenatal clinic of the Hospital's Central "Dr. Urquinaona", Maracaibo, Venezuela, between January 2012 and March 2018. The study protocol was approved by the Hospital Ethics Committee prior to the start of the research and written consent was obtained from all patients.
Pregnant women with a diagnosis of polyhydramnios, third trimester hemorrhage (placental abruption, placenta previa), suspected intrauterine growth restriction of the fetus (head circumference, abdominal circumference and femur length less than the 10th percentile reference with postnatal confirmation of weight less than the 10th percentile reference), severe preeclampsia with multiorgan manifestations were excluded; also fetuses with heart rate alterations, multiple gestation, presence of intrauterine or active maternal infection, chronic hypertensive disease (hypertension before 20 weeks of pregnancy), cardiac, hematologic, hepatic, renal or chronic systemic disease, pregestational or gestational diabetes mellitus, smoking, and those pregnant women in whom blood samples could not be obtained were not included. Patients who refused to participate in the research were also excluded.
Preeclampsia was established as systolic blood pressure of 140 mmHg or more or diastolic blood pressure of 90 mmHg or more, confirmed by 6 h or more difference. Blood pressure was measured in a sitting position after 15 min rest using a standard mercury sphygmomanometer with a 14-cm cuff. Systolic and diastolic blood pressure (taken relative to the fifth Korotkoff noise) was located relative to the nearest 2 mmHg point. The palpatory method was used to verify auscultatory readings of systolic blood pressure. Systolic and diastolic blood pressures were calculated from the average blood pressure of each arm. Proteinuria was defined as 300 mg or more of protein in a 24-h urine sample after 20 weeks of gestation.
Once the patients were selected for the study, a data collection form was filled out that included: patient identification, personal and gynecological-obstetrical history, prenatal control, gestational age (by date of last menstrual period or first trimester ultrasound) and leukocyte count values. Gestational age was calculated on the date of the last menstrual period and corrected by ultrasound if measurements during the first trimester showed a difference of more than 7 days. All pregnancies were followed until delivery and were categorized according to the development of preeclampsia (cases; group A) or not (controls; group B).
Antecubital vein blood samples were collected at the time of routine ultrasound evaluation in all selected pregnant women during the second trimester of pregnancy (17-20 weeks). Total and differential leukocyte counts were measured using an Abbott Cell-Dyn 3700 automated hematology analyzer (Abbott Laboratory®, USA). Absolute counts (cells x 103/mL) of cells were used in the analyses. NLR was determined using these values.
The values obtained were presented as mean +/standard deviation. The Kolmogorov-Smirnov test was used to test the normal distribution of the data (p > 0.05). Student's t test for unrelated samples was used for cluster analysis and to compare continuous variables. The accuracy of leukocyte, neutrophil, lymphocyte and NLR values for predicting the development of preeclampsia is presented in terms of sensitivity, specificity, positive predictive value and negative predictive value. Operator-receptor analysis was used to determine the best cutoff value. This was determined by the highest sensitivity and specificity represented by the highest Youden index p < 0.05 was considered statistically significant.
RESULTS
Measurement results were obtained for 504 primigravid pregnant women, of whom 41 patients (8.1%) developed preeclampsia (group A) and 463 pregnant women (91.9%) were considered controls (group B). The general characteristics of the 2 groups of pregnant women are shown in Table 1. No statistically significant differences were found in maternal age, gestational age, and systolic and diastolic blood pressure at the time of ultrasound (p = ns). Gestational age at diagnosis of preeclampsia in group A was 35.0 +/3.2 weeks. Statistically significant differences were observed in gestational age at delivery, systolic and diastolic blood pressure at delivery, 24-hour proteinuria, and newborn birth weight between the 2 groups of pregnant women (p < 0.0001).
Table 1 leukocyte count and neutrophil/lymphocyte ratio in the second trimester of pregnancy as a predictor of preeclampsia. general characteristics.
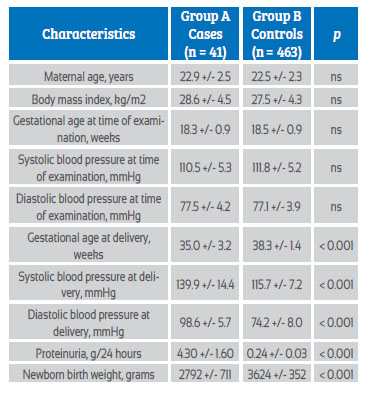
Table 2 shows the mean values of leukocyte count and NLR values. It was observed that patients in group A had significantly higher values of leukocytes (8.76 +/1.34 cells x 103/mL compared to8.32 +/1.21 cells x 103/mL; p = 0.0231) and neutrophils (6.61 +/1.30 cells x 103/mL compared to 5.67 +/1.44 cells x 103/mL; p < 0.0001) compared to group B patients. However, patients in group A had lower lymphocyte values (1.43 +/0.44 cells x 103/mL) compared to patients in group B (2.05 +/0.62 cells x 103/mL; p < 0.0001). NLR values were significantly higher in group A patients (4.70 +/1.25) compared to group B patients (2.82 +/1.20; cells x 103/mL; p < 0.0001).
Table 2 leukocyte count and neutrophil/lymphocyte ratio in the second trimester of pregnancy as a predictor of preeclampsia. comparison in each study group.
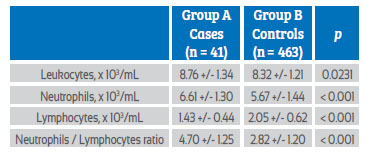
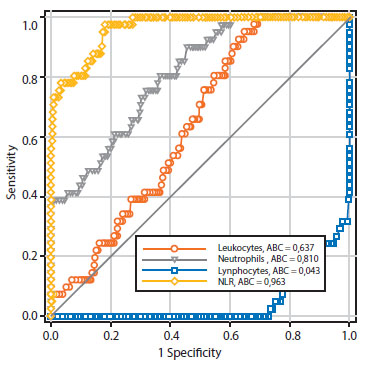
Table 3 and Figure 1 show the precision values for the prognosis of preeclampsia of the leukocyte count and NLR elements. Only the neutrophil (area under the curve of 0.810) and NLR (area under the curve of 0.963) values were shown to have area under the curve values that were useful for discriminating between patient groups for the development of preeclampsia. However, the NLR cutoff value of 3.3 was shown to have a higher sensitivity (97.5% compared with 90.2%), specificity (79.4% compared with 53.3%), positive negative predictive value (28.9% compared with 14.6%), negative predictive value (99.7% compared with 98.4%), and prognostic accuracy (81.0% compared with 56.3%) than the absolute neutrophil count cutoff value of 5.80 cells x 103/ mL.
Table 3 leukocyte count and neutrophil/lymphocyte ratio in the second trimester of pregnancy as a predictor of preeclampsia. prognostic efficacy.
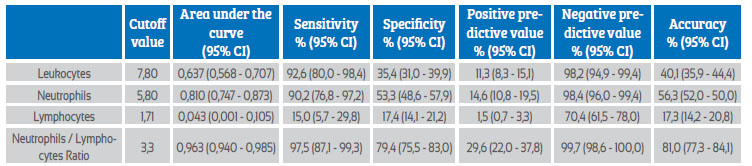
95% ci = 95% confidence interval.
DISCUSSION
Research results show that absolute values of neutrophils and NLR can be useful in the prediction of preeclampsia, as they have a good discriminatory capacity. However, NLR values had a higher predictive ability compared to neutrophil values. It has been proposed that higher circulating neutrophils and decreased lymphocytes are indicators of risk for cardiovascular events8. The proposed utility of elevated NLR values is that it combines the predictive ability of two leukocyte subtypes into a single risk factor9.
Pregnancy is a controlled inflammatory condition. The physiologic inflammatory activation that occurs in normal pregnancy is excessively increased in preeclampsia. The most widely accepted abnormalities in the pathophysiology of preeclampsia include endothelial dysfunction, impaired angiogenesis, and low-grade inflammation. Increased concentrations of proinflammatory cytokines produce free radical generation and oxidative stress, leading to endothelial injury10. Several factors have been suggested to be involved, including inflammatory cell activation and possible immunological changes, in which both neutrophils and lymphocytes participate, releasing cytokines and autoantibodies11. There is evidence that some markers of systemic inflammatory response obtained from routine peripheral blood hematology samples, such as NLR, have prognostic and predictive value in benign and malignant diseases, such as gynecological malignancies and inflammatory diseases in which alterations in immune cell counts are observed12,13.
Maternal circulating leukocytes are activated in pregnancy and their activity is even higher in preeclampsia14. Therefore, these activated cells could be responsible for the vascular dysfunction associated with the syndrome(15). Macrophages in the atherosclerotic plaque have a role as foam cells, whereas lymphocytes are part of the adaptive immune system producing antibodies16. Neutrophils are the first line of defense against infection at the site of injury, but they also infiltrate systemic vascular tissue in preeclamptic women, causing vascular inflammation(7). In preeclampsia they are likely to be activated in the intervillous space when exposed to oxidized lipids secreted by the placenta17. In addition, neutrophils obtained from preeclamptic women express significantly more cyclooxygenase-2, which regulates the release of thromboxane, tumor necrosis factor-alpha and superoxide, than those obtained from normotensive pregnant women or healthy nonpregnant women(18). However, the mechanisms responsible for these changes are not fully understood19.
Canzoneri et al.(20 found that the total leukocyte count was significantly increased in severe preeclamptic women compared to mild preeclamptic and normal pregnant women. This increase in total leukocyte count was mainly due to an increase in the number of neutrophils. It has been reported that the number of neutrophils increases 2.5-fold at 30 weeks of gestation during normal pregnancy and increases even more in preeclamptic women, with no significant differences in monocyte and basophil counts, accompanied by a decrease in absolute lymphocyte count compared to uncomplicated pregnant women21. This increase in the number of neutrophils during pregnancy may be the result of increased concentrations of colony-stimulating factors and circulating arachidonic acid22,23.
It has been considered that NLR could be a marker for predicting the onset and severity of preeclampsia. Although this research demonstrated that its determination can be useful in predicting the onset and development of the syndrome, other studies have reported contrary results regarding both its diagnostic and predictive capacity7,24-28). Two studies found that the NLR value was higher in preeclamptic women compared to controls but found no significant differences between the groups25,27. These results were contrary to the results of the present investigation. Other investigations have shown that NLR in preeclamptic women was significantly higher compared to controls(24,26,28). Similarly, there is evidence that the values could predict disease severity26). It has also been suggested that the increased NLR value in preeclamptic patients represents an independent predictor of disease severity28).
There is evidence proposing NLR as a prognostic factor for cardiovascular disorders since significant differences in the increase of this ratio are associated with increased risk of cardiovascular morbidity and mortality29. For this reason, it is considered that this inexpensive and noninvasive hematologic marker may be important for risk evaluation and prediction of preeclampsia, as well as a possible prognostic factor for future cardiovascular disease. Further research should be conducted to establish the role of NLR in other inflammatory conditions of pregnancy.
CONCLUSION
The neutrophil/lymphocyte ratio and absolute neutrophil count in the second trimester of pregnancy are useful tools in the prediction of preeclampsia, as patients who develop the syndrome have significantly higher concentrations in that trimester compared to healthy pregnant controls. However, further studies are needed to confirm these findings.
REFERENCES
1. Tomimatsu T, Mimura K, Endo M, Kumasawa K, Kimura T. Pathophysiology of preeclampsia: an angiogenic imbalance and long-lasting systemic vascular dysfunction. Hypertens Res. 2017;40(4):305-10. doi: 10.1038/hr.2016.152 [ Links ]
2. Lei T, Qiu T, Liao W, Li K, Lai X, Huang H, et al. Proteinuria may be an indicator of adverse pregnancy outcomes in patients with preeclampsia: a retrospective study. Reprod Biol Endocrinol. 2021;19(1):71. doi: 10.1186/s12958-021-00751-y [ Links ]
3. Gaudino M, Crea F. Inflammation in coronary artery disease: Which biomarker and which treatment? Eur J Prev Cardiol. 2019;26(8):869-71. doi: 10.1177/2047487319829307 [ Links ]
4. Lu HQ, Hu R. The role of immunity in the pathogenesis and development of pre-eclampsia. Scand J Immunol. 2019;90(5):e12756. doi: 10.1111/sji.12756 [ Links ]
5. Templeton AJ, Rodríguez-Lescure Á, Ruíz A, Alba E, Calvo L, Ruíz-Borrego M, et al. Prognostic role for the derived neutrophil-to-lymphocyte ratio in early breast cancer: a GEICAM/9906 substudy. Clin Transl Oncol. 2018;20(12):1548-56. doi: 10.1007/s12094-018-1885-5 [ Links ]
6. Liu J, Li S, Zhang S, Liu Y, Ma L, Zhu J, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33(8):e22964. doi: 10.1002/jcla.22964 [ Links ]
7. Liu N, Guo YN, Gong LK, Wang BS. Advances in biomarker development and potential application for preeclampsia based on pathogenesis. Eur J Obstet Gynecol Reprod Biol X. 2020;9:100119. doi: 10.1016/j.eurox.2020.100119 [ Links ]
8. Kithcart AP, Libby P. Unfriendly fire from neutrophils promiscuously potentiates cardiovascular inflammation. Circ Res. 2017;121(9):1029-31. doi: 10.1161/CIRCRESAHA.117.311867 [ Links ]
9. Taurino M, Aloisi F, Del Porto F, Nespola M, Dezi T, Pranteda C, Rizzo L, Sirignano P. Neutrophil-to-Lymphocyte ratio could predict outcome in patients presenting with acute limb ischemia. J Clin Med. 2021;10(19):4343. doi: 10.3390/ jcm10194343 [ Links ]
10. Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15(5):275-289. doi: 10.1038/s41581-0190119-6 [ Links ]
11. Opichka MA, Rappelt MW, Gutterman DD, Grobe JL, McIntosh JJ. Vascular dysfunction in preeclampsia. Cells. 2021;10(11):3055. doi: 10.3390/cells10113055 [ Links ]
12. Lee YH, Song GG. Neutrophil-to-lymphocyte ratio, mean platelet volume and platelet-to-lymphocyte ratio in Behçet's disease and their correlation with disease activity: A meta-analysis. Int J Rheum Dis. 2018;21(12):2180-7. doi: 10.1111/1756-185X.13404 [ Links ]
13. Li L, Tian J, Zhang L, Liu L, Sheng C, Huang Y, et al. Utility of preoperative inflammatory markers to distinguish epithelial ovarian cancer from benign ovarian masses. J Cancer. 2021;12(9):2687-93. doi: 10.7150/jca.51642 [ Links ]
14. Walsh SW, Strauss JF 3rd. The road to low-dose aspirin therapy for the prevention of preeclampsia began with the placenta. Int J Mol Sci. 2021;22(13):6985. doi: 10.3390/ijms22136985 [ Links ]
15. Feng X, Liu Y, Zhang Y, Zhang Y, Li H, Zheng Q, Li N, Tang J, Xu Z. New views on endothelial dysfunction in gestational hypertension and potential therapy targets. Drug Discov Today. 2021;26(6):1420-36. doi: 10.1016/j.drudis.2021.03.001 [ Links ]
16. Socha MW, Malinowski B, Puk O, Wartega M, Stankiewicz M, Kazdepka-Zieminska A, et al. The role of NF-?B in uterine spiral arteries remodeling, insight into the cornerstone of preeclampsia. Int J Mol Sci. 2021;22(2):704. doi: 10.3390/ijms22020704 [ Links ]
17. Chiarello DI, Abad C, Rojas D, Toledo F, Vázquez CM, Mate A, et al. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim Biophys Acta Mol Basis Dis. 2020;1866(2):165354. doi: 10.1016/j.bbadis.2018.12.005 [ Links ]
18. Miková E, Hrdý J. The role of neutrophils in preeclampsia. Ceska Gynekol. 2020;85(3):206-13. [ Links ]
19. Green S, Politis M, Rallis KS, Saenz de Villaverde Cortabarria A, Efthymiou A, Mureanu N, et al. Regulatory T cells in pregnancy adverse outcomes: A systematic review and meta-analysis. Front Immunol. 2021;12:737862. doi: 10.3389/fimmu.2021.737862 [ Links ]
20. Canzoneri BJ, Lewis DF, Groome L, Wang Y. Increased neutrophil numbers account for leukocytosis in women with preeclampsia. Am J Perinatol. 2009;26(10):729-32. doi: 10.1055/s-0029-1223285 [ Links ]
21. Lurie S, Frenkel E, Tuvbin Y. Comparison of the differential distribution of leukocytes in preeclampsia versus uncomplicated pregnancy. Gynecol Obstet Invest. 1998;45(4):229-31. doi: 10.1159/000009973 [ Links ]
22. Hayashi M, Ohkura T, Inaba N. Elevation of serum macrophage colony-stimulating factor before the clinical manifestations of preeclampsia. Am J Obstet Gynecol. 2003;189(5):1356-60. doi: 10.1067/s0002-9378(03)00674-4 [ Links ]
23. Mackay VA, Huda SS, Stewart FM, Tham K, McKenna LA, Martin I, et al. Preeclampsia is associated with compromised maternal synthesis of long-chain polyunsaturated fatty acids, leading to offspring deficiency. Hypertension. 2012;60(4):1078-85. doi: 10.1161/HYPERTENSIONAHA.112.197897 [ Links ]
24. Zheng WF, Zhan J, Chen A, Ma H, Yang H, Maharjan R. Diagnostic value of neutrophil-lymphocyte ratio in preeclampsia: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2019;98(51):e18496. doi: 10.1097/MD.0000000000018496 [ Links ]
25. Yücel B, Ustun B. Neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, mean platelet volume, red cell distribution width and plateletcrit in preeclampsia. Pregnancy Hypertens. 2017;7:29-32. doi: 10.1016/j.preghy.2016.12.002 [ Links ]
26. Serin S, Avci F, Ercan O, Köstü B, Bakacak M, Kiran H. Is neutrophil/lymphocyte ratio a useful marker to predict the severity of pre-eclampsia? Pregnancy Hypertens. 2016;6(1):22-5. doi: 10.1016/j.preghy.2016.01.005 [ Links ]
27. Yavuzcan A, Caglar M, Ustün Y, Dilbaz S, Ozdemir I, Yildiz E, et al. Mean platelet volume, neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in severe preeclampsia. Ginekol Pol. 2014;85(3):197-203. [ Links ]
28. Cakmak HA, Dincgez Cakmak B, Abide Yayla C, Inci Coskun E, Erturk M, Keles I. Assessment of relationships between novel inflammatory markers and presence and severity of preeclampsia: Epicardial fat thickness, pentraxin-3, and neutrophil-to-lymphocyte ratio. Hypertens Pregnancy. 2017;36(3):233-9. doi: 10.1080/10641955.2017.1321016 [ Links ]
29. Curran FM, Bhalraam U, Mohan M, Singh JS, Anker SD, Dickstein K, et al. Neutrophil-to-lymphocyte ratio and outcomes in patients with new-onset or worsening heart failure with reduced and preserved ejection fraction. ESC Heart Fail. 2021;8(4):3168-79. doi: 10.1002/ehf2.13424 [ Links ]
Statement of ethical issues
Ethical responsibilities: Protection of persons. The authors declare that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Confidentiality of data: The authors declare that they have followed the protocols of the Central Hospital "Dr. Urquinaona" and the University of Zulia on the publication of patient data.
Right to privacy and informed consent: The authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is held by the corresponding author.
Funding: The authors certify that they have not received financial support, equipment, in working personnel or in kind from individuals, public and/ or private institutions for the conduct of the study.
Cite as: Reyna-Villasmil E, Torres-Cepeda D, Mejía-Montilla J, Reyna-Villasmil N, RondónTapia M, Fernández-Ramírez A. Leukocyte count and neutrophil/lymphocyte ratio in the second trimester of pregnancy as a predictor of preeclampsia. Rev Peru Ginecol Obstet. 2022;68(2). DOI: https://doi.org/10.31403/rpgo.v68i2410
Received: December 10, 2021; Accepted: February 25, 2022











 texto en
texto en